|
| Mammography. Radiopaedia |
Causes of Breast Cancer and Strategies to Reduce Risk
Breast cancer is the most
common cancer diagnosis and the second leading cause of cancer death
among women in the United States. Each year, approximately 180,000
women will be diagnosed with the disease, and about 40,000 will die from
it (1).
Due to the dominance of breast cancer among women, this “Knol” focuses
on the role of lifestyle and pharmacologic strategies with
anti-estrogens in prevention of female breast cancer.
Incidence rates of breast cancer increased in the United States during most of the twentieth century. Over the last fifty years, incidence rates have also been rising in many other regions of the world, with the most notable increases in traditionally low-incidence Asian countries (2, 3). These international trends may reflect secular changes in reproductive patterns and lifestyle factors that affect breast cancer risk. In the USA, mortality rates have begun to decline since the early 1990s, in part due to improvements in screening practices and treatment effectiveness(4). A more recent dramatic decline in breast caner incidence reflects the substantial drop in the number of women taking postmenopausal hormone therapy after the results of the Women’s Health Initiative showed these drugs cause breast cancer (5).
This rapid decline in breast cancer in recent years (6, 7) together with migrant studies and substantial international variation in the incidence of disease, point to the enormous potential we have to prevent breast cancer. Additional evidence comes from randomized controlled clinical trials of anti-estrogens (discussed below), which show a 50% or greater reduction in new cases of breast cancer among women taking the active drug (Tamoxifen or Raloxifene) in the clinical trials (8, 9). This is the highest level of scientific evidence that a preventive intervention significantly reduces the onset of invasive and noninvasive breast cancers.


Mutations in the PTEN gene are responsible for Cowden’s disease, a syndrome characterized by hamartomas and benign lesions of the skin and oral cavity along with an increased risk of breast cancer. Thirty to 50 percent of women with Cowden’s disease are estimated to develop breast cancer by the age of 50 (18).
Ataxia telangiectasia (AT) is an autosomal recessive disease characterized by neurodegeneration, cerebral ataxia, oculo-cutaneous telangiectasia, sensitivity to radiation, and a 100-fold increased risk of developing cancer compared to the general population (19). The most common cancers among AT patients are lymphomas and leukemias, although solid tumors including breast cancer are included. Women heterozygous for mutations in the ataxia telangiectasia mutated (ATM) gene, estimated to be approximately one percent of the population, are reported to have a four to five-fold increased risk of breast cancer compared to non-carriers of the mutations (19-21), although not all studies have confirmed this association (22, 23).

Recent decline in new cases of breast cancer

Increasing evidence indicates that higher intake of folate is associated with reduced breast cancer risk (71, 72). Furthermore, women with higher folate intake appear to be protected from the increase in risk observed with alcohol (73), discussed below.
Fruits and vegetables are the major sources of intake for many of these nutrients, although fortified breakfast cereal and vitamin supplements are increasing as sources. There is some evidence that intake of fruits and vegetables may be protective against breast cancer. One review examined 70 different associations regarding particular fruits and vegetables and groups of fruits and vegetables in 21 epidemiologic studies. Most of those associations suggested some risk reduction (67). A combined reanalysis of data from eight prospective cohort studies that included more than 350,000 women, however, observed no evidence that intake of either fruits or vegetables reduces the risk of breast cancer (74). The effect of fruit and vegetable intake on risk, therefore, remains inconclusive.
In intervention studies, consumption of approximately two alcoholic drinks per day increased total and bioavailable estrogen levels in both premenopausal and postmenopausal women (77, 78), and single doses of alcohol acutely increased plasma estradiol levels in postmenopausal women (79), suggesting a mechanism by which alcohol may increase breast cancer risk. In prospective analyses, high intake of folic acid and high plasma folate levels appear to mitigate completely the excess risk of breast cancer associated with alcohol intake (72, 73, 80). Because alcohol metabolites inactivate folic acid, and low folate levels are associated with increased misincorporation of uracil into DNA, this finding suggests another mechanism for the adverse effects of alcohol.
Alcohol consumption has a complex mix of desirable and adverse health effects, one being an increase in breast cancer risk. Individuals should make decisions considering all the risks and benefits, but for a middle-aged women who drinks alcohol on a daily basis, reducing intake is one of relatively few behavioral changes that is likely to reduce risk of breast cancer. Taking a multiple vitamin containing folic acid greatly reduces risks of neural tube defects and may prevent coronary heart disease (81) and colon cancer (82), and growing evidence suggests this may mitigate the excess risk of breast cancer due to alcohol (73). Thus, taking a multiple vitamin appears sensible for women who do elect to drink regularly.
Soy foods have been extensively
investigated for potential protection against a range of chronic
conditions, including breast and prostate cancer, heart disease,
osteoporosis and menopausal symptoms. While the biologic components of
soy foods have been evaluated for their physiologic effects, to date
evidence suggests minimal effect of soy intake on female hormones
(estrogens) in pre and postmenopausal women but potential reduction in
LH and FSH among premenopausal women (87).
Despite little impact of intake on hormone levels, studies nevertheless
suggest higher intakes of soy are associated with reduced menopausal
symptoms (88).
Cardiovascular protection could be mediated through the fat content of soy (20% of energy from fat), which is predominantly polyunsaturated (89). Studies relating soy intake to heart disease suggest a reduction in risk in blood pressure, lipids and insulin levels with higher soy intake (90).
Interest in soy and cancer risk is motivated in part by historically low breast and prostate cancer risk among Asians. Detailed review by Wu and colleagues shows that at high intakes typical of Asian diets soy intake is significantly related to reduced risk for breast cancer, and the effect may be strongest for intake in childhood and adolescence. Combining data from numerous studies they found that intake of high amounts of soy (20 mg per day of isoflavone) in Asian women was associated with a decreased risk for breast cancer, compared to Asian women consuming lower amounts (5 mg daily) (91). However, even the lowest intake of soy isoflavones in the Asian population was more than fivefold the “high” intake (0.8 mg per day) of women in Western countries, where studies have not shown a protective effect for soy.
In sum, little evidence of adverse effects is seen in the literature and potential substantial benefits may be obtained with intakes that currently exceed typical consumption in the US.
A modest inverse relation between body weight (typically used as body mass index, BMI, calculated as weight in kilograms divided by height in meters2, to account for variation in height) and incidence of premenopausal breast cancer has been consistently observed in both case-control and cohort studies (97). Heavier premenopausal women, even at the upper limits of what are considered to be healthy weights, have more irregular menstrual cycles and increased rates of anovulatory infertility (98), suggesting that their lower risk may be due to fewer ovulatory cycles and less exposure to ovarian hormones.
In both case-control and prospective studies conducted in affluent Western countries, the association between BMI and risk of breast cancer among postmenopausal women has been only weakly positive (55, 96). The lack of a stronger association has been surprising because obese postmenopausal women have plasma levels of endogenous estrogens nearly twice as high as lean women. However, an elevated body mass index in a postmenopausal woman represents two opposing risks: a protective effect due to the correlation between early weight and postmenopausal weight, and an adverse effect due to elevated estrogens after menopause. For this reason, weight gain from early adult life to after menopause should be more strongly related to postmenopausal breast cancer risk than attained weight, and this has been consistently supported by both case-control (99) and prospective studies (100-102). Another reason for failing to appreciate a greater adverse effect of excessive weight or weight gain on risk of postmenopausal breast cancer is that the use of postmenopausal hormones obscures the variation in endogenous estrogens due to adiposity and elevates breast cancer risk regardless of body weight. Among women who never used postmenopausal hormones in the Nurses’ Health Study, those who gained 25 kilograms or more after age 18 had double the risk of breast cancer compared with women who maintained their weight within two kilograms (101). In 2002 the International Agency for Research on Cancer convened a committee to evaluate weight, activity, and cancer prevention. After thoroughly reviewing the evidence they concluded that overweight and obesity causes postmenopausal breast cancer and that current levels of obesity in the US cause approximately 10% of postmenopausal breast cancer. These cases could be avoided if adult weight gain was avoided.
Incidence rates of breast cancer increased in the United States during most of the twentieth century. Over the last fifty years, incidence rates have also been rising in many other regions of the world, with the most notable increases in traditionally low-incidence Asian countries (2, 3). These international trends may reflect secular changes in reproductive patterns and lifestyle factors that affect breast cancer risk. In the USA, mortality rates have begun to decline since the early 1990s, in part due to improvements in screening practices and treatment effectiveness(4). A more recent dramatic decline in breast caner incidence reflects the substantial drop in the number of women taking postmenopausal hormone therapy after the results of the Women’s Health Initiative showed these drugs cause breast cancer (5).
This rapid decline in breast cancer in recent years (6, 7) together with migrant studies and substantial international variation in the incidence of disease, point to the enormous potential we have to prevent breast cancer. Additional evidence comes from randomized controlled clinical trials of anti-estrogens (discussed below), which show a 50% or greater reduction in new cases of breast cancer among women taking the active drug (Tamoxifen or Raloxifene) in the clinical trials (8, 9). This is the highest level of scientific evidence that a preventive intervention significantly reduces the onset of invasive and noninvasive breast cancers.
Effect of age
Data from incidence of breast cancer around the world show that
risk accumulates rapidly from menarche to menopause and then much more
slowly after menopause. See figure below for age incidence curve in USA.
 |
| Source: NCHS/SEER (click to enlarge) |
The rapid increase in risk from menarche to first birth, at
some 8.5% per year is further exacerbated by the societal changes, with
menarche coming earlier with industrialization and age at first birth
being delayed with increasing equality in education opportunities for
women. With industrialization we have exacerbated the physiologic
exposure of the breast to adverse effects of hormones that drive breast
cancer risk. As risk accumulates up to menopause and then the rate of
further increase slows dramatically, modifying risk accumulation early
in life could have greatest pay off. (See soy intake discussion
below). Radiation data from the follow-up of women exposed to the
atomic bomb in Japan clearly show that exposure in childhood and
adolescence carries a far greater adverse effect on breast cancer risk
than exposure later in life.
Family History and Genetics
A history of breast cancer in the family has been known to increase
risk for women for more than 100 years. Studies in the UK in the late
1800s showed that breast cancer was more common in families in which a
woman had been diagnosed with breast cancer. Either a history of breast
cancer diagnosed in a mother or sister increases risk about 2 to 3 fold
compared to women without family history. The younger a family member is
when diagnosed with breast cancer, the higher the risk among other
family members (12). On average, five to ten percent of breast cancers are due to inherited genetic mutations.
Some genetic factors that contribute to this family history are
particularly strong and carry even higher risk with them. These genetic
inherited risks include the BRCA1 and 2 genes and rare inherited risks
such as Li-Fraumeni syndrome.
BRCA1 and 2 are more common among Ashkenazi Jewish women though
this genetic alteration is observed in non-Jewish women too. Women who
carry the BRCA1 gene have a lifetime risk for beast cancer – that is a
40 to 80 percent chance of being diagnosed with beast cancer in their
lifetime. Two to five percent of all breast cancers are estimated to be
attributable to germline mutations in BRCA1 and/or BRCA2 (13, 14). Research has indicated that these two genes are involved in genome stability, DNA repair, and cell cycle checkpoint control (15). Women of Ashkenazi Jewish ancestry or from Iceland or Poland are more likely to harbor mutations in the BRCA genes (16).
Evidence now clearly shows that removal of both ovaries substantially
reduces risk of breast cancer among women who carry the BRCA1 gene by
approximately 50% (17).
Other genes contributing to
breast cancer include p53 which is a tumor suppressor gene associated
with hereditary breast cancer. Li-Fraumeni syndrome is a rare cancer
syndrome linked to mutations in p53. Individuals with this syndrome are
at increased risk of leukemias and cancers of the lung, brain and
breast. The prevalence of germline mutations in p53 are relatively rare
and thus do not contribute to a large portion of breast cancers. Mutations in the PTEN gene are responsible for Cowden’s disease, a syndrome characterized by hamartomas and benign lesions of the skin and oral cavity along with an increased risk of breast cancer. Thirty to 50 percent of women with Cowden’s disease are estimated to develop breast cancer by the age of 50 (18).
Ataxia telangiectasia (AT) is an autosomal recessive disease characterized by neurodegeneration, cerebral ataxia, oculo-cutaneous telangiectasia, sensitivity to radiation, and a 100-fold increased risk of developing cancer compared to the general population (19). The most common cancers among AT patients are lymphomas and leukemias, although solid tumors including breast cancer are included. Women heterozygous for mutations in the ataxia telangiectasia mutated (ATM) gene, estimated to be approximately one percent of the population, are reported to have a four to five-fold increased risk of breast cancer compared to non-carriers of the mutations (19-21), although not all studies have confirmed this association (22, 23).
Low-penetrance genes
There is a great deal of evidence to suggest that other genes with
low penetrance may also affect breast cancer susceptibility. Low
penetrance genes are expected to confer only a small amount of risk, but
because the variation is likely to be more common, the population
attributable risk for these genetic polymorphisms, alone or in
combination with other risk factors, is likely to be high. Recent
studies have included advanced methods to scan the whole genome for
genetic changes that may convey increased risk of breast cancer. While
new markers of risk have been identified to date these do not show
application for any one of these to either demarcate risk or offer
strategies for prevention. In fact it is estimated that only 7 women in a
million will carry all of the common low risk genetic markers for
breast cancer. Some studies have suggested that the higher the number of
genetic changes a woman has the higher her risk for beast cancer. While
such findings may help stratify risk within the population, to date
clinical applications using these markers have not been developed.
Preliminary math from the UK and US suggest that the range of relative
risk defined by the presence or absence of each of the 7 genetic changes
indentified as related to breast cancer through genome wide studies
gives only a 2 fold range of risk (24).
Furthermore, the proportion of the population of women carrying two
copies of all 7 high-risk genetic markers is tiny (25 women in 35
million US women ages 50 to 79).
Hormones
The
common feature of female reproductive hormones around the world is the
monthly cycle of estrogen, progesterone, and leutenizing hormone.
Ovarian hormones play a central role in breast cancer etiology. Both
those produced in the body and those taken as pills increase the
proliferation of breast tissue, thereby increasing the likelihood of
random genetic errors during cell division. Many of the established
risk factors – including early onset of first period, late menopause,
and being overweight or obese after menopause – contribute to the
cumulative “dose” of estrogen for the breast. Obesity and hormones taken
for relief of menopausal symptoms are major sources of exposure among
postmenopausal women. Across the life course, reproductive variables
play a major role in setting the level of risk a woman has for breast
cancer. Few of these reproductive risk events such as timing of first
birth, are modifiable in light of existing societal norms; though they
change rapidly in populations as they progress though economic
transition. Among postmenopausal women the major sources of circulating
estrogens are either pills or hormones produced from fat cells. Higher
levels of body fat correlate with higher circulating hormone levels and
these levels lead directly to higher risk of breast cancer. Thus
focusing on exposure to estrogens among postmenopausal women remains a
high priority for prevention.
Circulating estrogens
Circulating estrogens
Growing
evidence shows a strong and consistent link between circulating
estrogen and testosterone levels in the blood among postmenopausal women
and their risk of developing breast cancer. The combined prospective
data show that the positive relation between circulating hormone levels
and breast cancer is dominant and independent of a woman’s level of
obesity and other risk factors (10).
In the updated analysis form the Nurses’ Health Study there is a three
to four-fold increased risk comparing top to bottom quarter of the
population according to their hormone levels. This increase in risk is
strongest for breast tumors that are classified as estrogen receptor
positive (11).
Exogenous Hormones
Exogenous Hormones
Oral contraceptives
It was originally believed that oral contraceptives might increase
breast cancer risk, since they contain concentrations of estrogen and
progestin that could be greater than the levels of these hormones
produced by a woman during a normal ovulatory cycle (25).
Results of more than 50 studies have provided considerable reassurance
that there is little, if any, increase in risk with oral contraceptive
use in general, even among women who have used oral contraceptives for
ten or more years. However, current users and recent users (fewer than
ten years since last use) have a modest elevation in risk compared to
never users. In a combined reanalysis including more than 53,000 cases
of breast cancer, the relative risk for current users compared to never
users was 1.24, (a 24% increase in risk compared to nonusers of the same
age) while the relative risks for women one to four years after
stopping and five to nine years after stopping were 1.16 and 1.07,
respectively (26).
A recent national US study showed no increase in risk among current
users, perhaps reflecting changes in formulation from the earlier
studies (27).
Because most women taking oral contraceptives are young and, therefore,
are at low absolute risk, even a modest increase in risk will result in
few additional cases of breast cancer. For example, the increase in
risk among 10,000 women ages 16 to 19 using the OC for 5 years, risk
through 10 years after stopping is approximately 4.5 cases in the 10,000
women compared to 4 cases if the 10,000 women had not used OCs (one
half excess case in 10,000 women). For use from age 25 to 29, 4.7 excess
cases of breast cancer would be diagnosed among 10,000 women. A
typical figure reproduced from the Lancet is set out below to show the
excess risk for use from ages 25 to 29.
Postmenopausal hormones
The relation between postmenopausal estrogen use and risk of breast
cancer has been investigated in many epidemiologic studies over the
past 30 years. While initial studies did not address formulation of
hormone therapy used, substantial advances in the past decade have
clarified the impact of differing patterns of hormone use on risk of
postmenopausal breast cancer. Increased risk has been observed in two
important subgroups: users of long duration, and current users; although
the magnitude of risk varies according to use of estrogen alone, or
estrogen plus progestin.
In a large, reanalysis that combined data from 51 epidemiologic
studies, the investigators observed a statistically significant
association between current or recent use of predominantly unopposed
estrogen and risk of breast cancer, with the strongest positive
association among those with the longest duration of use (28).
Among women who had used postmenopausal hormones within the previous
five years (compared to never users of postmenopausal hormones), the
relative risks for duration of use were 1.1 for one to four years, 1.3
for five to nine years, 1.2 for ten to 14 years, and 1.6 for 15 years or
more of use. No significant increase in breast cancer risk was noted
for women who had quit using postmenopausal hormones five or more years
in the past, regardless of their duration of use. Of note this increase
in risk was stronger among lean women than among obese women who would
already have higher circulating hormone levels even before taking
postmenopausal hormone therapy.
In the Breast Cancer Detection Demonstration Project cohort
(BCDDP), a positive association with invasive breast cancer was noted
among current users of five to 15 or more years duration (29).
An underlying concern is that these data are not independent of
duration of use; at any age, past users will have accumulated a shorter
duration of use of postmenopausal hormones than continuing current
users. The randomized controlled trial within the Women’s Health
Initiative among women who had a hysterectomy showed no increase in risk
of invasive breast cancer over the 9 years of follow-up (30).
In a comparable analysis of the Nurses’ Health Study, Chen and
colleagues showed that risk of breast cancer was not elevated until
after 10years of use, consistent with the Women’s Health Initiative
(WHI) and that after 20 years of use of unopposed estrogen the risk of
breast cancer was 1.42 (95%CI 1.05-2.07). In this study risk was higher
for estrogen receptor positive tumors. Data on how recently a woman has
used hormones and risk of breast cancer is sparse because many earlier
studies did not distinguish current from past users. In the report from
the Nurses’ Health Study cohort (31), an excess risk of breast cancer was limited to women with current or very recent use of postmenopausal hormones.
The addition of a progestin to estrogen regimens became
increasingly common through the 1980s to 2000, as it minimizes or
eliminates the increased risk of endometrial hyperplasia and endometrial
cancer associated with using unopposed estrogens. The impact of an
added progestin on the risk of breast cancer has been evaluated
rigorously only in the last fifteen years. Two of the first studies to
assess this relationship suggested that the addition of a progestin
could decrease breast cancer risk (32, 33).
However, these studies were small, and potentially important
confounders (e.g., age, parity) were not accounted for in the analyses.
Numerous additional studies have assessed this relationship and
together indicate that a protective effect of typical doses used in
postmenopausal hormone therapy can be ruled out (28, 34-36).
Consistent with the epidemiologic evaluations noted above, the WHI
showed a significant increase in risk of breast cancer among women
taking estrogen plus progestin and that risk increased with increasing
duration of use (37, 38).
As seen in the report from the Million Women Study in the United
Kingdom, the relative risk of breast cancer for current users of
estrogen only preparations compared to never users was 1.30 (95% CI =
1.22-1.38), while the relative risk for current users of estrogen plus
progestin combinations was 2.00 (95% CI = 1.91-2.09); this observed
difference in the magnitudes of the associated risk was highly
significant (39),
and consistent with other epidemiologic findings and with the WHI
findings. The result from the WHI underestimates the adverse effect of
combination estrogen plus progestin as the women in this randomized
trial in large part stopped taking the drug during the follow-up (more
than 40% of women on the active drug) but were counted in the primary
analysis as though they had continued using the drug. Importantly,
recent data from the WHI show that breast cancer mortality is also
elevated among women who have used estrogen plus progestin (40).
Because widespread use of estrogen plus progestin is so recent, few
data are currently available to evaluate the effect of different
formulations, doses, or schedules of use of progestin on risk of breast
cancer. The results from the Million Women Study, however provide the
largest range of information and indicate little variation in risk based
on specific doses of estrogen or regimens, including oral or patch
administration (39).
Postmenopausal hormone use involves a complex trade-off of benefits
and risks. From the standpoint of breast cancer risk reduction, the
optimal strategy would be to use estrogens not at all, or at most for a
few years to relieve menopausal symptoms. Added progestins in particular
should be used for a limited time, if at all.
Recent decline in new cases of breast cancer
A decline in incidence of breast cancer has been reported in the
US, New Zealand, and other countries since the early stopping of the WHI
trial of estrogen plus progestin – which happened in part due to the
significant increase in risk for beast cancer.
Based on data from the San Francisco mammography registry,
prescribing of estrogen plus progestin, the active drug in the WHI
trial, peaked in 1999. Before publication of the Heart and
Estrogen/Progestin Replacement Study (HERS), the use of hormone therapy
was increasing at 1% per quarter, but declined by 1% per quarter after
the publication (41).
This decline in prescribing continued until the publication of the WHI
in 2002, at which point a more substantial decline of 18% per quarter
was observed. The peak and decline through 1999 to 2002 is concordant
with the HERS report (42)
in 1998 showing a significant increase in CHD in the first year of
therapy among women with prevalent coronary disease, and in addition, no
long-term benefit in reducing CHD (43).
The growing epidemiologic evidence published since 2000 on the adverse
effects of combination therapy on breast cancer added further evidence
against the use of this therapy.
Evidence for breast cancer incidence rates now clearly shows a
parallel drop in breast cancer consistent with the pattern of decreased
prescribing. The rigorous, state-of-the-art analysis by Jemal et al (4)
drawing on SEER incidence data from 1975 through 2003 — shows that
there is a significant decrease in incidence of invasive breast cancer
from 1999 to 2003 in all 5-year age groups from 45 years and above, and a
sharp decrease largely limited to ER positive tumors in age groups 50
to 69 between 2002 and 2003. Furthermore, while others have suggested
that a 1 to 3 percent drop in screening mammography may account for this
drop in incidence, Jemal shows strong evidence against this.
Furthermore an analysis within the San Francisco mammography cohort
evaluated only women who had completed mammography and a decline in
breast cancer of similar magnitude was observed (44). Thus a decrease in screening cannot explain the decline in incidence.
Based on these data and a through review of scientific evidence the
International Agency for Research on Cancer has concluded that
combination estrogen plus progestin is carcinogenic to human (45).
Thus women should avoid this combination of drugs whenever possible. In
addition, unopposed estrogen increases risk for beast cancer, with risk
increasing as the duration of use increases. Furthermore, this increase
in risk is greatest and also most clearly seen among lean women – who
have low circulating estrogen levels due to their lean body mass.
Anti-estrogens
The potential for prevention of breast cancer through drug therapies is supported by results from randomized trials of SERMs (8, 9, 46, 47).
Both tamoxifen and raloxifene have been shown to reduce the incidence
of invasive breast cancer by approximately 50%, with the benefit largely
limited to ER+ tumors, where risk is reduced by as much as 80%. Adverse
effects of tamoxifen suggest that the potential use for chemoprevention
will be limited to a subset of women at increased risk and younger in
age, in large part because of increasing incidence of adverse effects
with age (48).
The adverse effects experienced in the 8 year randomized trial of
raloxifene (Continuing Outcomes Relevant to Evista (CORE)), on the other
hand, are somewhat fewer than those observed for tamoxifen(47).
Of note, there was no statistically significant difference in overall
mortality or uterine cancer among women randomized to Raloxifene
compared to placebo. While Raloxifene is approved in the United States
for use to prevent osteoporosis in postmenopausal women (49), and a number of cost effectiveness studies support this use in conjunction with screening for osteoporosis (50-52).
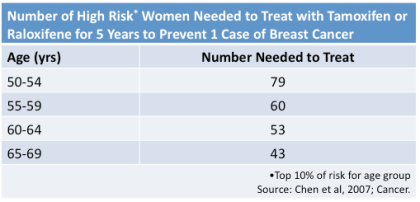
We calculated some numbers to help women decided (53).
Among women in the top 10 percent of breast cancer risk in each 5-year
age group we estimated how many women would need to take a SERM for 5
years to prevent one case of breast cancer. Thee numbers are summarized
in the table below reproduced from Chen, et al., Cancer 2007.
Physicians will need to play a key role in advising women in this
rapidly evolving field.
Nutritional Factors
Dietary fat
The relationship between fat intake
and breast cancer risk has been the focus of a large number of studies
and has received substantial public attention. High fat diets have long
been known to increase the occurrence of mammary tumors in rodents, but
fat consumption has been confounded by energy intake in many animal
experiments, rendering the interpretation of these data difficult. High
energy intake regardless of composition also increases risk of breast
and other cancers in rodents. The dietary fat hypothesis is largely
based on the observation that national per capita fat consumption is
highly correlated with breast cancer mortality rates (54).
A serious problem with this international comparison of diet and breast
cancer, however, is the potential for confounding by known and
suspected breast cancer risk factors (e.g., low parity, late age at
first birth) that have vastly different distributions among regions of
the world.
Studies that identify women diagnosed
with breast cancer and get them to recall their past diet have
accounted for confounding by total energy intake. These studies have
indicated a weak positive association between fat intake and breast
cancer risk. Howe et al. (55)
conducted a meta-analysis to summarize the results from twelve smaller
case-control studies comprising a total of 4,312 cases and 5,978
controls. The overall pooled relative risk for a 100-gram increase in
daily total fat intake was 1.35, and the risk was somewhat stronger for
postmenopausal than premenopausal women. However, because the average
total fat consumption is about 70 grams per day for U.S. women, a
reduction in fat intake as large as 100 grams would be impossible for
almost all women. Furthermore, relative risks of this magnitude in
case-control studies may easily be due to selection bias (the controls
are drawn from a population with a different distribution of fat intake
than the distribution in the population that gave rise to the cases) or
recall bias (the cases, knowing their diagnosis, differentially
misreport their pre-diagnosis diet) (56).
Prospective cohort studies should not
be subject to these biases, or distortion in results, because the
population that gives rise to the cases is known and dietary information
is collected before the onset of disease. A reanalysis has been
conducted of all the prospective studies, including a total of 4,980
cases of breast cancer among 337,819 women (57).
Overall, no association was observed between intake of total,
saturated, monounsaturated, or polyunsaturated fat and risk of breast
cancer, and no reduction in risk was seen even for fat intakes as low as
20 percent of energy. The Women’s Health Initiative randomize
controlled trial comparing reduction in dietary fat intake and risk of
breast cancer observed no significant reduction in risk over the average
8.1 years of the trial (58).
Women reduced their fat intake from 37.8% of energy at baseline to 30%
on average, and the modest, 9% lower rate of breast cancer in the
intervention group was not statistically significant. The women on the
intervention or low fat diet lost weight and the control women eating
their normal diet gained weight, making interpretation of the results
difficult as weight loss in women after menopause is related to lower
risk of breast cancer. This confirms the prospective epidemiologic
evidence that modification of total dietary fat intake is unlikely to
reduce breast cancer risk.
On the other hand, some findings
have indicated that specific types of fat could differentially affect
risk of breast cancer. In most animal studies, diets high in
polyunsaturated fat (linoleic acid), but typically at levels beyond
human exposure, have clearly increased the occurrence of mammary tumors,
but a positive association has not been found in prospective
epidemiologic studies (57).
In contrast, high intake of omega-3 fatty acids from marine oils has
inhibited the occurrence of mammary tumors in animals, but case-control
and cohort studies generally have found little relation between intake
of omega-3 fatty acids or fish (the major source of extra long chain
omega-3 fatty acids) and risk of breast cancer (59).
Some animal studies have suggested that monounsaturated fat, in the
form of olive oil, may be protective relative to other sources of energy
(60),
and several epidemiologic studies have supported these findings. For
example, in a Spanish study specifically undertaken because of the high
consumption of olive oil and low breast cancer rates in this population,
no association was observed with total fat intake, but higher intake of
olive oil was associated with reduced risk of breast cancer; women in
the highest quartile of consumption had approximately 35 percent lower
risk compared to women in the lowest quartile (61).
Similar inverse associations with olive oil or monounsaturated fat were
seen in case-control studies in Greece, Italy, and elsewhere in Spain (59).
In a recent report from the Nurses’ Health Study II, high intake of
animal fat, but not vegetable fat, in early adulthood was associated
with elevated breast cancer risk (62).
As noted by the investigators, however, a biologic mechanism to explain
this observed association remains to be elucidated, and other
components in food containing animal fat (e.g., heterocyclic amines,
fat-soluble hormones or growth factors) could be responsible.
Fiber
Fiber has been hypothesized to lower
breast cancer risk. Fiber inhibits re-absorption of estrogens in the
gastrointestinal tract (63),
which may lead to lower circulating levels of estrogens, and a high
fiber diet has been associated with reduced incidence of mammary tumors
in animals (60). Case-control studies originally suggested a moderate protective effect of fiber (55). Prospective studies, however, have shown little or no association between fiber intake and breast cancer risk (64-66).
Micronutrients and fruits and vegetables
Vitamins A, C, and E and carotenoids have been examined in relation to breast cancer risk. These nutrients function as antioxidants, neutralizing free radicals that can cause DNA damage. There is little evidence of an association of retinol (preformed vitamin A) with risk, with the exception of a possible effect of intake from supplements. For b-carotene intake, most but not all studies have found that risk decreases with increasing intakes (67), and studies of blood levels of carotenoids also suggest decreasing risk with higher levels (68, 69). Higher intakes of vitamins C and E, on the other hand, do not appear to be protective (70).Increasing evidence indicates that higher intake of folate is associated with reduced breast cancer risk (71, 72). Furthermore, women with higher folate intake appear to be protected from the increase in risk observed with alcohol (73), discussed below.
Fruits and vegetables are the major sources of intake for many of these nutrients, although fortified breakfast cereal and vitamin supplements are increasing as sources. There is some evidence that intake of fruits and vegetables may be protective against breast cancer. One review examined 70 different associations regarding particular fruits and vegetables and groups of fruits and vegetables in 21 epidemiologic studies. Most of those associations suggested some risk reduction (67). A combined reanalysis of data from eight prospective cohort studies that included more than 350,000 women, however, observed no evidence that intake of either fruits or vegetables reduces the risk of breast cancer (74). The effect of fruit and vegetable intake on risk, therefore, remains inconclusive.
Alcohol
The association between alcohol consumption and breast cancer risk has been evaluated in more than 100 investigations that now clearly support a causal relation. In a pooled analysis of the six cohort studies with data on alcohol and dietary factors that included 200 or more cases (75), the risk of breast cancer increased monotonically with increasing intake of alcohol, with no statistical evidence of heterogeneity among studies; the multivariate relative risk for a ten-gram per day increase in alcohol was 1.09 (95% CI = 1.04 – 1.13). Beer, wine and liquor all contribute to the positive association (67, 75), strongly suggesting that alcohol per se is responsible for the increased risk. One study has shown that recent adult drinking may be more important than drinking patterns earlier in life and that reductions in consumption in mid-life should reduce risks of breast cancer (76).In intervention studies, consumption of approximately two alcoholic drinks per day increased total and bioavailable estrogen levels in both premenopausal and postmenopausal women (77, 78), and single doses of alcohol acutely increased plasma estradiol levels in postmenopausal women (79), suggesting a mechanism by which alcohol may increase breast cancer risk. In prospective analyses, high intake of folic acid and high plasma folate levels appear to mitigate completely the excess risk of breast cancer associated with alcohol intake (72, 73, 80). Because alcohol metabolites inactivate folic acid, and low folate levels are associated with increased misincorporation of uracil into DNA, this finding suggests another mechanism for the adverse effects of alcohol.
Alcohol consumption has a complex mix of desirable and adverse health effects, one being an increase in breast cancer risk. Individuals should make decisions considering all the risks and benefits, but for a middle-aged women who drinks alcohol on a daily basis, reducing intake is one of relatively few behavioral changes that is likely to reduce risk of breast cancer. Taking a multiple vitamin containing folic acid greatly reduces risks of neural tube defects and may prevent coronary heart disease (81) and colon cancer (82), and growing evidence suggests this may mitigate the excess risk of breast cancer due to alcohol (73). Thus, taking a multiple vitamin appears sensible for women who do elect to drink regularly.
Soy and phytoestrogens
Much public interest currently
focuses on the potential for phytoestrogens to reduce the risk of breast
cancer. Phytoestrogens are naturally occurring plant compounds that may
alter estrogen metabolism away from genotoxic metabolites. However,
several intervention studies show no evidence to support a protective
role for phytoestrogens from soy. For example, in a study in which women
consumed 38 grams of soy protein daily for five months, premenopausal
women experienced elevated plasma estradiol concentrations and no change
in progesterone (83).
Of concern, however, was that 29.2 percent of the women had epithelial
hyperplasia on nipple aspirate during the months they were consuming soy
protein. Growing evidence suggests that hyperplasia in nipple aspirate
may be a useful marker for risk of breast cancer. In another study,
women with benign or malignant breast disease who were randomized to a
60-gram soy supplement showed a significant increase in the
proliferation rate of breast cells on biopsy, another potential marker
of breast cancer risk, after only 14 days of soy supplementation (84), and similar results were seen in the normal breast tissue of premenopausal women (85).
In contrast, a large prospective study in Japan with 427 cases of
incident breast cancer demonstrated no relation between the intake of
soy products in 1970 and the risk of subsequent breast cancer during
approximately 500,000 person-years of follow-up (86).
Given the potential for adverse effects, a priority must be to clarify
the relation between phytoestrogen intake and breast cancer risk.
Cardiovascular protection could be mediated through the fat content of soy (20% of energy from fat), which is predominantly polyunsaturated (89). Studies relating soy intake to heart disease suggest a reduction in risk in blood pressure, lipids and insulin levels with higher soy intake (90).
Interest in soy and cancer risk is motivated in part by historically low breast and prostate cancer risk among Asians. Detailed review by Wu and colleagues shows that at high intakes typical of Asian diets soy intake is significantly related to reduced risk for breast cancer, and the effect may be strongest for intake in childhood and adolescence. Combining data from numerous studies they found that intake of high amounts of soy (20 mg per day of isoflavone) in Asian women was associated with a decreased risk for breast cancer, compared to Asian women consuming lower amounts (5 mg daily) (91). However, even the lowest intake of soy isoflavones in the Asian population was more than fivefold the “high” intake (0.8 mg per day) of women in Western countries, where studies have not shown a protective effect for soy.
In sum, little evidence of adverse effects is seen in the literature and potential substantial benefits may be obtained with intakes that currently exceed typical consumption in the US.
Vitamin D
Epidemiologic evidence on dietary intake and also studies of blood vitamin D levels and risk of disease are inconclusive (92). Only two studies have evaluated blood levels of vitamin D at diagnosis and survival after breast cancer. The first, published last year included 512 cases of breast cancer followed for an average of 11.6 years (93). 116 women developed distant recurrence and 106 died during follow-up. This study showed an increase in risk of distant recurrence and death among those with low vitamin D levels. New data from the WHEL study of over 3,000 women with breast cancer identified 518 women with new breast cancer events during an average of 7.3 years of follow-up (94). In this substantially larger study, there was no evidence for a trend in risk with level of vitamin D overall, or when pre and postmenopausal women were evaluated separately. Despite these two studies the overall level of evidence remains inconclusive with limited events to inform these analyses.Body Size
Height
Epidemiologic studies in a variety of populations have found that height is positively related to breast cancer risk. In a pooled analysis of seven prospective cohort studies (95), the relative risk for each five-centimeter increase in height, after controlling for other breast cancer risk factors, was 1.07 for all women (95% CI = 1.02-1.11). The relative risk for women 1.75 meters (approximately 69 inches) or taller compared to those 1.60 meters (about 63 inches) or shorter was 1.42 for premenopausal women and 1.28 for postmenopausal women. Attained height is determined by a mixture of genetic and environmental factors, with one environmental determinant being childhood energy intake (96). The association between height and breast cancer risk appears to be stronger in populations where childhood growth was limited by energy deprivation, which suggests that energy intake early in life may play a role in breast carcinogenesis. Clearly this is not a modifiable risk factor.Weight and weight change during adulthood
Attained weight and weight change in adults summarize the balance between long-term energy intake and expenditure. The relation between adiposity and breast cancer depends on menopausal status: in affluent Western populations with high rates of breast cancer, measures of body fatness are inversely related to risk of premenopausal breast cancer, and body fatness is positively related to postmenopausal breast cancer risk.A modest inverse relation between body weight (typically used as body mass index, BMI, calculated as weight in kilograms divided by height in meters2, to account for variation in height) and incidence of premenopausal breast cancer has been consistently observed in both case-control and cohort studies (97). Heavier premenopausal women, even at the upper limits of what are considered to be healthy weights, have more irregular menstrual cycles and increased rates of anovulatory infertility (98), suggesting that their lower risk may be due to fewer ovulatory cycles and less exposure to ovarian hormones.
In both case-control and prospective studies conducted in affluent Western countries, the association between BMI and risk of breast cancer among postmenopausal women has been only weakly positive (55, 96). The lack of a stronger association has been surprising because obese postmenopausal women have plasma levels of endogenous estrogens nearly twice as high as lean women. However, an elevated body mass index in a postmenopausal woman represents two opposing risks: a protective effect due to the correlation between early weight and postmenopausal weight, and an adverse effect due to elevated estrogens after menopause. For this reason, weight gain from early adult life to after menopause should be more strongly related to postmenopausal breast cancer risk than attained weight, and this has been consistently supported by both case-control (99) and prospective studies (100-102). Another reason for failing to appreciate a greater adverse effect of excessive weight or weight gain on risk of postmenopausal breast cancer is that the use of postmenopausal hormones obscures the variation in endogenous estrogens due to adiposity and elevates breast cancer risk regardless of body weight. Among women who never used postmenopausal hormones in the Nurses’ Health Study, those who gained 25 kilograms or more after age 18 had double the risk of breast cancer compared with women who maintained their weight within two kilograms (101). In 2002 the International Agency for Research on Cancer convened a committee to evaluate weight, activity, and cancer prevention. After thoroughly reviewing the evidence they concluded that overweight and obesity causes postmenopausal breast cancer and that current levels of obesity in the US cause approximately 10% of postmenopausal breast cancer. These cases could be avoided if adult weight gain was avoided.
Weight loss in adult years and after menopause has been studies in a
limited fashion, in part due to the low number of women who loose
weight and avoid regaining it. Recent prospective data from the Nurses’
Health Study show that weight loss after menopause is related to reduced
risk of breast cancer, and the risk reduction is greatest for estrogen
receptor positive tumors (103). Women who loose 10 or more kilograms and maintain the weight loss have a 40% reduction in their risk of breast cancer.
Avoiding weight gain during adult life can importantly reduce risk of
postmenopausal breast cancer as well as cardiovascular disease and many
other important conditions. Individual women can reduce weight gain by
exercising regularly and moderately restraining caloric intake. Health
care providers play an important role in counseling patients throughout
adult life about the importance of weight control.
 |
| Source: Eliassen et al, 2006. (Click to enlarge) |
Physical Activity
The relation of physical activity to risk of breast cancer has been
assessed by the International Agency for Research on Cancer, which
concluded that, although studies have not been entirely consistent, the
overall results support a reduction in risk with higher levels of
activity (104).
Evidence for a dose-response effect was found in most of the studies
that examined the trend. The majority of studies have focused on
postmenopausal breast cancer, although there is also some evidence for a
protective effect of physical activity on premenopausal disease.
Importantly, recent evidence shows the benefit of activity is present
regardless of race or ethnicity (105).
The strongest protection against breast cancer has been reported from
studies showing consistent high levels of activity from menarche through
adult life (106, 107).
Activity through adult life at the level of 4 hours or more of walking
per week appears to be sufficient to offer protection against breast
cancer. Women in the most active group through adolescence and adult
years are at 35 percent lower risk for beast cancer.
In general, it is recommended that adults engage in 30 minutes of
activity each day. This level of activity appears sufficient to lower
risk of breast cancer. The incorporation of greater physical activity
into daily life will be difficult for many persons unless governments
provide a safer and more accessible environment for pedestrians and
bicycle riders. The provision of worksite and community exercise
facilities can also contribute importantly. Health care providers can
counsel and reinforce increasing activity as a health lifestyle choice
that lowers risk of breast cancer and improves the risk profile for a
range other chronic conditions.
Cigarette Smoking
The Surgeon General’s report of 2004 on the Health Consequences of
Smoking reviewed the overall evidence on smoking and breast cancer risk
and concluded that the evidence is suggestive of no causal relation
between active smoking and breast cancer. Thus although cessation from
smoking will not modify risk of breast cancer, physicians should counsel
all smokers to stop smoking to avoid the broad range of adverse health
consequences of this additive behavior.
Reproductive Factors
Age at menarche and characteristics of the menstrual cycle
Menarche, the first menstrual period a young woman has, represents
the development of the mature hormonal environment for a woman and the
onset of monthly cycling of hormones that induce ovulation, then monthly
menstrual period, and cell proliferation within the breast and also the
lining of the uterus (endometrium). Earlier age at first menstrual
period is consistently associated with increased risk of breast cancer,
and most studies suggest that age at menarche is related to both
premenopausal and postmenopausal breast cancer (108). Breast cancer risk generally decreases by ten to 20 percent with each one-year delay in menarche (25).
To date no meaningful interventions to modify age a menarche have been
identified. Over the past 150 years age at menarche has decreased
around the world with industrialization, improved childhood nutrition
and fewer childhood infections.
Although menarche is most clearly related to the onset of
ovulation, new evidence shows that hormone levels during the
premenopausal years increase risk of breast cancer. Hankinson et al.
evaluate risk of breast cancer among a cohort of over 30,000 women who
had given timed blood samples and been followed for incidence of breast
cancer. Both estrogen and testosterone levels among these premenopausal
women were independently related to increased risk of premenopausal
breast cancer (see below).
Parity, age at first full-term pregnancy, and lactation
Nulliparous women (those who have had no children) are at greater
risk of breast cancer compared to women who have had one or more
children. This increased risk is evident for breast cancer diagnosed
after age 40 to 45 years, but not for breast cancer occurring at younger
ages. A younger age at first full-term pregnancy predicts a lower
lifetime risk of breast cancer (108).
This is in part due to the final maturation of the breast with the
hormones that circulate during the first pregnancy in preparation for
breast feeding. The reduction in risk of breast cancer following
pregnancy is not immediate, but rather takes approximately 10 to 15
years (109).
In fact, risk of breast cancer is increased for the first decade
following first pregnancy, with a greater adverse effect the older the
age of the woman at firsts birth and the longer the interval from
menarche to the first birth (110-112).
A higher number of births also lowers risk of breast cancer; each
additional birth beyond the first reduces long-term risk of breast
cancer. The more closely subsequent births are spaced the lower the
lifetime risk of breast cancer (111, 113).
While the patterns of these reproductive factors in the population have
continued to change over time, the pattern of age at first birth and
spacing of subsequent births is largely driven by social factors
including education of women, career advances, and family support
systems. As a consequence they are not considered as modifiable risk
factors for prevention of breast cancer.
As early as 1926, it was proposed that a breast never used for lactation is more liable to become cancerous (114).
The overall evidence supports a reduction in risk with longer duration
of breastfeeding. The combined evidence from the Oxford collaborative
group reanalysis of case-control and cohort studies indicates that
independent of parity, lactation is consistently related to reduced risk
(115). The relative risk of breast cancer decreases by 4.3 percent for every 12 months of breastfeeding.
Spontaneous and induced abortion
A number of studies have examined the relationship between
spontaneous and induced abortion and breast cancer risk. Results from
epidemiologic studies have been inconsistent (108).
By far the strongest study to date on the association between breast
cancer and abortion was a population-based cohort study made up of 1.5
million Danish women born between 1935 and 1978 (116).
Of these women, 18.4 percent had had one or more induced abortions.
After adjusting for potential confounders, the risk of breast cancer for
women with a history of induced abortion was the same as the risk for
women who had no history of induced abortion. Results from this
population-based prospective cohort provide strong evidence against an
increase in risk of breast cancer among women with a history of induced
abortion during the first trimester. Taken as a whole, the available
evidence does not support any important relation between induced
abortion and risk of breast cancer.
Age at menopause
The rate of increase in breast cancer incidence slows at menopause,
which marks the termination of the monthly cycling of hormones that
induce regular breast cell proliferation. Early studies of age at
menopause showed that women who undergo bilateral oophorectomy at a
young age have a greatly reduced risk of breast cancer (117, 118). On average, the risk of breast cancer increases by some three percent per year of delay in age at menopause (119).
The effect of artificial menopause by either bilateral oophorectomy or
pelvic irradiation appears to be somewhat greater than the effect of
natural menopause, due to the immediate cessation of ovarian function
rather than a gradual decline over months or years (25). Evidence indicates that age at natural menopause has been stable over centuries.
Precursor Neoplastic Lesions
Benign breast disease (BBD) includes a number of breast
abnormalities. These benign conditions vary in their cellular and
pathologic features and, most importantly, in their impact on subsequent
breast cancer risk. Three clinically most relevant groups are defined
by changes in breast cells include: non-proliferative, proliferative
without atypia, and proliferative with atypia (120).
Non-proliferative lesions include cysts, apocrine metaplasia, and
mild hyperplasia of usual type. Women with these lesions are at the same
risk of breast cancer as women without a breast biopsy (120).
Proliferative lesions without atypia (e.g., intraductal papilloma,
sclerosing adenosis, moderate hyperplasia of usual type) are associated
with a 1.5 to 2-fold increased risk of breast cancer compared to
non-proliferative lesions (120, 121).
Atypical ductal (ADH) and lobular (ALH) hyperplasias make up the group
of proliferative lesions with atypia. Atypical hyperplasias are similar
to in situ carcinomas in that they are both characterized by
proliferation of epithelial cells, but they do not share all of the
morphologic and pathologic features. These lesions are associated with a
3.5 to 6 fold increased risk of subsequent breast cancer (122).
A large follow-up of 9087 women for a median of 15 years by
investigators at Mayo Clinic showed that risk was greater among women
diagnosed prior to menopause and that there was no interaction between
histologic findings and family history of breast cancer (123).
Other risk factors such as alcohol and hormone use do not appear to act
differently according to types of benign breast disease, indicating
that prevention strategies apply across women with and without a history
of benign lesions.
Molecular Genetic Characteristics of Tumor
Hormone receptor status
The effects of estrogen and progesterone on cell growth and
development are mediated through hormone receptors. The majority of
breast cancer tumors express estrogen (ER) and progesterone (PR)
receptors. The ER and PR status of the cancer is important for two
reasons. First, tumors that express these receptors at high levels tend
to be more differentiated, and these patients are likely to have a
better prognosis. Second, ER and PR expression is strongly predictive
of the tumors’ response to hormonal or anti-estrogen therapies. Risk
factor patterns differ according to receptor status and indicate that
the receptors are markers of different tumor types rather than stages of
a single disease with a single disease pathway (124).
While the observed adverse effect of first pregnancy appears to drive
ER negative tumors, this type of added insight into etiology of subtypes
of breast caner does not yet inform prevention strategies.
Risk factors in early life and adolescence
The majority of research on determinants of breast cancer risk has
focused on risk factors in adulthood, but animal data and epidemiologic
evidence now suggest that exposures in earlier periods of life may have
important effects on risk. Mammary gland tissue exists in a partially
undifferentiated state throughout the perinatal period, rendering it
susceptible to carcinogenesis (125).
Trichopoulos proposed that high concentrations of maternal estrogens
during pregnancy in humans may increase the probability of breast cancer
in daughters by creating a “fertile soil” for subsequent cancer
initiation (126).
This hypothesis has been supported by epidemiologic studies showing
moderate positive associations between indicators of high prenatal
estrogen levels – such as birthweight, maternal age, and twin
pregnancies – and adult breast cancer risk; in contrast, pre-eclampsia
and eclampsia, indicators of low pregnancy estrogen levels, appear to be
inversely associated with risk (127a-c).
Furthermore, although little research has been conducted on exposures
shortly after birth, case-control studies have observed significant
reductions in risk among women who were breastfed as infants (128).
Findings from studies of in utero and perinatal exposures are
inconsistent, however, and specific biologic mechanisms to explain the
apparent associations remain unclear.
Growing evidence indicates that the years between menarche and
first birth are important in establishing future breast cancer risk (129).
During this time period, undifferentiated cells of the breast are
proliferating rapidly in response to ovarian hormones. In rats,
pregnancy and lactation induce terminal differentiation of cells, which
leads to lengthening of their average cell-cycling time and more time
for DNA repair; exposure to carcinogens after the first pregnancy
results in very few tumors (130).
Studies of atomic bomb survivors in Hiroshima have shown that exposure
to ionizing radiation is associated with increased breast cancer risk
and that the magnitude of the increase is dependent on age at exposure
as well as on dose; the younger women were at the time of the bombing,
the greater their excess risk (131).
Among girls who were treated with repeated fluoroscopy for tuberculosis
or with ionizing radiation for Hodgkin’s disease, younger age at
exposure to radiation also confers greater breast cancer risk (132, 133).
Lifestyle factors during early life may also affect breast cancer
risk. Greater body fatness during childhood and adolescence has been
associated with reduced breast cancer risk (134, 135), and proliferative benign breast disease (136),
and association that may be due to increased frequency of menstrual
irregularities and anovulatory cycles among overweight girls, or altered
hormone levels prior to menarche.
Certain dietary factors during adolescence also may affect risk of breast cancer (137) and benign breast disease (138).
For example, higher vitamin E and vegetable fat intake during high
school was related to lower risk of proliferative benign breast disease
confirmed by central pathology review, and with invasive premenopausal
breast cancer (139).
Although further research in this area is necessary to confirm these
findings, adolescence may constitute a major time period for breast
cancer prevention (129).
Mammographic density
The radiographic appearance of the breast on a mammogram varies
depending on the composition of the individual breast. Fat is
radiolucent and appears dark on mammogram, while epithelial cells and
connective tissue are radiodense and appear light. Mammographic density
can be measured continuously as the overall percentage of dense tissue
in the breast or with a categorical rating system. There is evidence
that women with the greatest mammographic densities are at a four to
six-fold increased risk of breast cancer compared to women with little
or no density (140, 141),
making mammographic density one of the strongest independent risk
factors for breast cancer. Likewise, radiologists reading the mammograms
can classify the reading according to the level of density, this also
predicts risk to the same magnitude as the more systematic and objective
research measures of density. It is unclear what the biologic mechanism
is for this relationship, although it has been hypothesized that
mammographic density is a marker for cellular proliferation in the
breast tissue (142). Several studies show that endogenous hormone levels do not drive mammographic density (143) or the risk for beast cancer associated with increased desnity(144).
Thus these two risk factors are independent predictors of risk. While
uptake of combination estrogen plus progestin increases breast density,
lifestyle interventions for reduction in density remain to be
identified.
Other Environmental Factors
Biologically persistent organochlorines have received considerable
attention as possible causes of breast cancer. These compounds include
pesticides (e.g., DDT), industrial chemicals (e.g., PCBs), and dioxins
produced as combustion products of PCBs or contaminants of pesticides.
While several small studies have evaluated possible relations, the
pooled analysis of data from five large studies in the northeastern
United States has found no association between PCBs and DDE levels and
breast cancer risk (145).
Overall, recent studies have not found evidence of increased risk of
breast cancer, and organochlorines appear unlikely to be major breast
cancer risk factors.
While much popular attention is focused on lifestyle factors such
as use of underarm deodorant or antiperspirant, which may contribute to
higher risk of breast cancer in westernized societies, a rigorous study
of this topic showed no association (146).
Numerous studies evaluating a possible relation between silicone
breast implants and risk of breast cancer have failed to show any
positive association. In fact, most observational studies have reported
lower rates of breast cancer among women with implants (147-150). Overall, these data provide strong evidence that breast implants do not lead to increased risk of breast cancer.
Risk Assessment
Breast cancer incidence models have also been applied to predict
individual probabilities of carrier status for specific mutations that
drive risk of breast cancer and alternatively, based on a varying number
of risk factors, to predict the risk of breast cancer over a defined
time period, say 5 or 10 years. The larger the number of risk factors
considered, the higher the likelihood the prediction model will separate
those at risk of disease form those who are not as likely to develop
disease. However, as Wald notes (151),
to be useful as a screening test or an individual marker of risk or to
identify those who will develop disease and those who will not, the
magnitude of association for a predictor must be in the order of 10 or
higher comparing extreme quintiles for a detection rate of 20%. No
prediction models for breast cancer have achieved this level of
discrimination to date.
Evaluation of an individual woman’s risk of breast cancer has
become much more important because this risk can now be modified. Until
recently, risk has been primarily based on an evaluation of family and
reproductive history and history of benign breast disease. New
information on risk based on detailed histologic characteristics of
benign breast disease (152), and serum hormone levels (153)
now has the potential to allow a much more powerful prediction of risk
for an individual woman. However, because risk factors may change over
the life course, (weight gain, change in alcohol intake, menopausal
status, use of postmenopausal hormones for some years, etc,) it becomes
more helpful to consider the impact of all these risk factors on breast
cancer cumulative risk up to a given age, say 70 or 75. This approach
has been developed for breast cancer risk according to family history (154), and the prediction of BRCA1 carrier status (155, 156), but more general applications joining carrier status and lifestyle factors remain limited (157).
The complex nature of breast cancer incidence, with many possibly
time dependent risk factors, requires prediction models that account for
this variation over time. These are now shown to outperform traditional
approaches that fit indicator variables with fixed effects across time (158).
In addition, the log-incidence model of Rosner and Colditz performs
significantly better than the commonly used Gail model for total breast
cancer incidence that includes only 5 variables (age, age at menarche,
age at first birth, number of benign breast biopsies, and family
history).
The efficacy of chemoprevention for breast cancer is clearly shown for ER+ disease reducing risk by 50% (8). Given the need to balance risks and benefits when implementing a Tamoxifen-or Raloxifene based chemoprevention strategy (48),
a model that successfully identifies women at increased risk of ER+
breast cancer will, therefore, improve the risk benefit ratio. Colditz
and Rosner have applied their log-incidence model to breast cancers
classified according to receptor status and reported that the area under
the ROC curve adjusted for age was 0.630 (95% CI = 0.616 to 0.644) for
ER+/PR+ tumors and was 0.601 (95% CI = 0.575 to 0.626) for ER-/PR-
tumors, indicating adequate discriminatory accuracy. On the other hand,
when we fitted the Gail model to the same data set it had performance
characteristics that were somewhat lower than the Rosner and Colditz
model with values of 0.578 for total cancer and 0.57 for ER+PR+ tumors.
The difference between the area under the ROC for the Rosner and Colditz
model vs. the Gail model for total breast cancer was statistically
significant (p < 0.0001) indicating that the more complete modeling
of risk factors across the life course could be more useful for
discriminating among those women at high and low risk of breast cancer.
Growing efforts are in place to add endogenous hormone levels and
mammographic density to models that rely on established epidemiologic
risk factors (159).
To date, addition of mammographic density has added little to the
performance of models as simple as the Gail model, increasing the area
under the ROC curve by just 1% (160).
In the future, screening for elevated estrogen levels in postmenopausal
women to help identify those who would most benefit from an estrogen
antagonist, as is done for serum cholesterol, may become part of medical
practice.
Summary
The available evidence provides a basis for a number of strategies
that can reduce risk of breast cancer, although some of these represent
complex decision making. Attainable objectives can make an important
impact on individual risk of breast cancer. However, the collective
implementation of all lifestyle strategies will not reduce population
rates of breast cancer to the very low levels of traditional poor
societies because the magnitude of the necessary changes is unrealistic
or undesirable.
For women avoiding weight gain in adult life and reduction in
excess weight after menopause significantly reduce risk of breast
cancer. Among both pre and postmenopausal women higher levels of
physical activity lower the risk of breast cancer.
Avoiding excess alcohol intake and including a multivitamin to
counter the adverse effects of alcohol will help avoid breast cancer
risk accumulation.
Given the evidence reviewed above, a role will exist for hormonal
and other chemopreventive interventions that may be appropriate for
women at particularly high risk and, potentially, for wide segments of
the population, as few women can be considered to have very low risk.
Because approaches such as chemoprevention carry risks and benefits it
is important to weigh up these factors. Thus the balance of risks and
benefits will break in favor of use among women at higher risk of breast
cancer. This may be up to 25% of women in the age range from 50 to 70.
We have set out one possible scenario, though others will soon be
developed to help women weigh up their risks and consider the benefits
of use.
Together, the modification of nutritional and lifestyle risk
factors and the judicious use of chemopreventive agents can have a major
impact on incidence of this important disease. Such strategies will
complement early detection through screening mammography programs to
reduce the mortality burden from breast cancer.
—————————————
Key Prevention Messages – 8 Ways to Prevent Breast Cancer
Women perceive breast cancer as one of their biggest health threats. Some simple lifestyle changes can help lower risk.
1. Keep weight in check
Women who maintain a healthy weight throughout adulthood have a lower risk of breast cancer, especially if they are post-menopausal. One reason is that fat tissue affects different hormone levels in the body. Too much fat tissue can lead to higher hormone levels and increase the risk of cancer. Weight loss after menopause lowers risk of breast cancer. It’s never too late to benefit from losing weight.2. Be physically active
People who are physically active for at least 30 minutes a day have a lower risk of breast cancer, possibly because physical activity affects hormone levels and other growth factors in the body. Being physically active is also one of the best ways to help maintain a healthy weight. In addition, physically active people also have a lower risk of colon cancer, heart disease, diabetes and stroke.3. Avoid too much alcohol
Women who have less than one drink a day have a lower risk of breast cancer. (One drink is a can of beer, a glass of wine, or a shot of hard liquor.) Alcohol may raise the level of some hormones in the body. High levels of certain hormones after menopause may cause cells in the breast to become cancerous.4. Take a daily multivitamin with folate
In general, there are no strong links between specific vitamins and the risk of breast cancer. However, in women who drink moderate amounts of alcohol, the vitamin folate (found in most multivitamins and B-complex vitamins) seems to protect against the increased risk associated with drinking alcohol. Women who breast feed for a total of one year or more have a lower risk of breast cancer. This is because breast feeding can cause changes in hormones and in breast tissue that help protect the cells from becoming cancerous. Women who regularly breast feed also have a lower risk of ovarian cancer.5. Breastfeed, if possible
Women who breast feed for a total of one year or more have a lower risk of breast cancer. This is because breast feeding can cause changes in hormones and in breast tissue that help protect the cells from becoming cancerous. Women who regularly breast feed also have a lower risk of ovarian cancer.6. Avoid birth control pills, particularly after age 35
Women currently on birth control pills have a higher risk of breast cancer. Yet, birth cont
rol
pills can have positive and negative effects on a woman’s health. If
taken for at least 5 years, birth control pills can lower a woman’s risk
of colon cancer, uterine cancer and ovarian cancer. But while she’s
taking them, they raise her risk of breast cancer, heart attack and
stroke.
7. Avoid postmenopausal hormones
Post-menopausal hormones are medications that help ease the symptoms of menopause, like hot flashes and vaginal dryness. Use over 1- 2 year, though, can increase the risk of breast cancer and other serious conditions, like heart disease.8. If at increased risk after menopause, consider a prescription anti-estrogen, like tamoxifen or raloxifene
Tamoxifen and raloxifene are medications prescribed for women at high risk of breast cancer. They block the effects of the hormone estrogen in breast tissue and can substantially reduce the risk of breast cancer. However, these medications also have serious side effects. They are not right for everyone and can only be prescribed by a doctor. Talk to your doctor if you have questions about your risk and whether these drugs may be right for you.
—————————————————————————————————-
Strategies to Avoid Weight Gain – Sustain Weight Loss
Physical Activity / Sedentary Behavior Goals- Brisk walking (or similar effort) for at least 20 minutes increasing up to 60 minutes, 6 days per week OR walking a total of 10,000 steps per day (building up to 10,000 if needed)
- Limit television to less than 2 hours per day.
- Do strength training exercises at least 2 days per week
- Replace sugary drinks with unsweetened choices (water, diet tea).
- Eat breakfast every day.
- Eat a diet rich in fruits and vegetables (8-10 svgs/d) and whole grain foods, like brown rice and whole wheat bread (at least 6 svgs/d).
- Drink alcohol in moderation, if at all (no more than 1 drink/d for women, 2/d for men).
- Log weight every day (at the same time every day)
- Exercise at the same time every day (like before work/school; during lunch).
- Keep portion sizes small and avoid seconds
- Avoid fast food restaurants. Choose healthier options if you need to, like a salad with fat-free dressing or a fruit cup.
Related links
Your Disease RiskKomen Foundation – About Breast Cancer
Literature cited
1. American Cancer Society. Cancer Facts and Figures, 2006. Atlanta: American Cancer Society; 2006 Contract No.: Document Number|.2. Nagata C, Kawakami N, Shimizu H. Trends in the incidence rate and risk factors for breast cancer in Japan. Breast Cancer Research and Treatment. 1997;44:75-82.
3. Seow A, Duffy S, McGee M, Lee J, Lee H. Breast cancer in Singapore: Trends in incidence 1968-1992. Int J Epidemiol. 1996;25:40-5.
4. Jemal A, Ward E, Thun MJ. Recent trends in breast cancer incidence rates by age and tumor characteristics among U.S. women. Breast Cancer Res. 2007;9(3):R28.
5. Colditz GA. Decline in breast cancer incidence due to removal of promoter: combination estrogen plus progestin. Breast Cancer Res. 2007;9(4):108.
6. Clarke CA, Glaser SL, Uratsu CS, Selby JV, Kushi LH, Herrinton LJ. Recent declines in hormone therapy utilization and breast cancer incidence: clinical and population-based evidence. J Clin Oncol. 2006;24(33):e49-50.
7. Robbins A, Clarke C. Regional changes in hormone therapy use and breast cancer incidence, California, 2001-2004. J Clin Oncol. 2007;25:3437-8.
8. Fisher B, Costantino J, Wickerham D, Redmond C, Kavanah M, Cronin W, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. 1998;90:1371-88.
9. Martino S, Cauley JA, Barrett-Connor E, Powles TJ, Mershon J, Disch D, et al. Continuing outcomes relevant to Evista: breast cancer incidence in postmenopausal osteoporotic women in a randomized trial of raloxifene. J Natl Cancer Inst. 2004;96(23):1751-61.
10. The Endogenous Hormones and Breast Cancer Collaborative Group. Endogenous sex hormones and breast cancer in postmenopausal women: reanalysis of nine prospective studies. J Natl Cancer Inst. 2002;94:606-16.
11. Missmer SA, Eliassen AH, Barbieri RL, Hankinson SE. Endogenous estrogen, androgen, and progesterone concentrations and breast cancer risk among postmenopausal women. J Natl Cancer Inst. 2004;96(24):1856-65.
12. Colditz GA, Willett WC, Hunter DJ, Stampfer MJ, Manson JE, Hennekens CH, et al. Family history, age, and risk of breast cancer. J Am Med Assoc. 1993;270:338-43.
13. Easton D, Ford D, Peto J. Inherited susceptibility to breast cancer. Cancer Surv. 1993;18:95-113.
14. Oesterreich S, Fuqua SA. Tumor suppressor genes in breast cancer. Endocr Relat Cancer. 1999;6(3):405-19.
15. Unger MA, Weber BL. Recent advances in breast cancer biology. Curr Opin Oncol. 2000;12(6):521-5.
16. Narod SA. Modifiers of risk of hereditary breast and ovarian cancer. Nat Rev Cancer. 2002;2(2):113-23.
17. Rebbeck TR, Kauff ND, Domchek SM. Meta-analysis of risk reduction estimates associated with risk-reducing salpingo-oophorectomy in BRCA1 or BRCA2 mutation carriers. J Natl Cancer Inst. 2009;101(2):80-7. PMCID: 2639318.
18. Foulkes WD, Rosenblatt J, Chappuis PO. The contribution of inherited factors to the clinicopathological features and behavior of breast cancer. J Mammary Gland Biol Neoplasia. 2001;6(4):453-65.
19. Swift M, Morrell D, Massey RB, Chase CL. Incidence of cancer in 161 families affected by ataxia-telangiectasia. N Engl J Med. 1991;325(26):1831-6.
20. Inskip HM, Kinlen LJ, Taylor AM, Woods CG, Arlett CF. Risk of breast cancer and other cancers in heterozygotes for ataxia-telangiectasia. Br J Cancer. 1999;79(7-8):1304-7.
21. Janin N, Andrieu N, Ossian K, Lauge A, Croquette MF, Griscelli C, et al. Breast cancer risk in ataxia telangiectasia (AT) heterozygotes: haplotype study in French AT families. Br J Cancer. 1999;80(7):1042-5.
22. FitzGerald MG, Bean JM, Hegde SR, Unsal H, MacDonald DJ, Harkin DP, et al. Heterozygous ATM mutations do not contribute to early onset of breast cancer. Nat Genet. 1997;15(3):307-10.
23. Chen J, Birkholtz GG, Lindblom P, Rubio C, Lindblom A. The role of ataxia-telangiectasia heterozygotes in familial breast cancer. Cancer Res. 1998;58(7):1376-9.
24. Pharoah PD, Antoniou AC, Easton DF, Ponder BA. Polygenes, risk prediction, and targeted prevention of breast cancer. N Engl J Med. 2008;358(26):2796-803.
25. Bernstein L. Epidemiology of endocrine-related risk factors for breast cancer. J Mammary Gland Biol Neoplasia. 2002;7(1):3-15.
26. Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and hormonal contraceptives: collaborative reanalysis of individual data on 53,297 women with breast cancer and 100,239 women without breast cancer from 54 epidemiological studies. Lancet. 1996;347:1713-27.
27. Marchbanks PA, McDonald JA, Wilson HG, Folger SG, Mandel MG, Daling JR, et al. Oral contraceptives and the risk of breast cancer. N Engl J Med. 2002;346(26):2025-32.
28. Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and hormone replacement therapy: collaborative reanalysis of data from 51 epidemiologic studies of 52,705 women with breast cancer and 108,411 women without breast cancer. Lancet. 1997;350:1047-59.
29. Schairer C, Byrne C, Keyl PM, Brinton LA, Sturgeon SR, Hoover RN. Menopausal estrogen and estrogen-progestin replacement therapy and risk of breast cancer (United States). Cancer Causes Control. 1994;5:491-500.
30. Stefanick ML, Anderson GL, Margolis KL, Hendrix SL, Rodabough RJ, Paskett ED, et al. Effects of conjugated equine estrogens on breast cancer and mammography screening in postmenopausal women with hysterectomy. Jama. 2006;295(14):1647-57.
31. Colditz GA, Hankinson SE, Hunter DJ, Willett WC, Manson JE, Stampfer MJ, et al. The use of estrogens and progestins and the risk of breast cancer in postmenopausal women. N Engl J Med. 1995;332:1589-93.
32. Nachtigall LE, Nachtigall RH, Nachtigall RD, Beckman EM. Estrogen replacement therapy, II: a prospective study in the relationship to carcinoma and cardiovascular and metabolic problems. Obstet Gynecol. 1979;54:74-9.
33. Gambrell RD, Jr., Maier RC, Sanders BI. Decreased incidence of breast cancer in postmenopausal estrogen-progestogen users. Obstet Gynecol. 1983;62:435-43.
34. Ross RK, Paganini-Hill A, Wan P, Pike M. Effect of hormone replacement therapy on breast cancer: estrogen versus estrogen plus progestin. JNCI. 2000;92:328-32.
35. Schairer C, Lubin J, Troisi R, Sturgeon S, Brinton L, Hoover R. Menopausal estrogen and estrogen-progestin replacement therapy and breast cancer risk. JAMA. 2000;283:485-91.
36. Newcomb PA, Titus-Ernstoff L, Egan K, Trentham-Dietz A, Baron J, Storer B, et al. Postmenopausal estrogen and progestin use in relation to breast cancer risk. Cancer Epidemiol Bio Prevention. 2002;11:593-600.
37. Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288(3):321-33.
38. Chlebowski RT, Hendrix SL, Langer RD, Stefanick ML, Gass M, Lane D, et al. Influence of estrogen plus progestin on breast cancer and mammography in healthy postmenopausal women: the Women’s Health Initiative Randomized Trial. Jama. 2003;289(24):3243-53.
39. Beral V. Breast cancer and hormone-replacement therapy in the Million Women Study. Lancet. 2003;362(9382):419-27.
40. Chlebowski RT, Anderson GL, Gass M, Lane DS, Aragaki AK, Kuller LH, et al. Estrogen plus progestin and breast cancer incidence and mortality in postmenopausal women. JAMA. 2010;304(15):1684-92.
41. Haas JS, Kaplan CP, Gerstenberger EP, Kerlikowske K. Changes in the use of postmenopausal hormone therapy after the publication of clinical trial results. Ann Intern Med. 2004;140(3):184-8.
42. Hulley S, Grady D, Bush T, Furberg C, Herrington D, Riggs B, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. Jama. 1998;280(7):605-13.
43. Grady D, Herrington D, Bittner V, Blumenthal R, Davidson M, Hlatky M, et al. Cardiovascular disease outcomes during 6.8 years of hormone therapy: Heart and Estrogen/progestin Replacement Study follow-up (HERS II). Jama. 2002;288(1):49-57.
44. Kerlikowske K, Miglioretti DL, Buist DS, Walker R, Carney PA. Declines in Invasive Breast Cancer and Use of Postmenopausal Hormone Therapy in a Screening Mammography Population. J Natl Cancer Inst. 2007.
45. International Agency for Research on Cancer. Combined estrogen-progestogen postmenopausal therapy. Combined Estrogen-progestogen Contraceptives and Combined Estrogen-progestogen Menopausal Therapy. Lyon, France: International Agency for Research on Cancer; 2007.
46. Cummings SR, Duong T, Kenyon E, Cauley JA, Whitehead M, Krueger KA. Serum estradiol level and risk of breast cancer during treatment with raloxifene. JAMA. 2002;287(2):216-20.
47. Vogel VG, Costantino JP, Wickerham DL, Cronin WM, Cecchini RS, Atkins JN, et al. Update of the National Surgical Adjuvant Breast and Bowel Project Study of Tamoxifen and Raloxifene (STAR) P-2 Trial: Preventing breast cancer. Cancer Prev Res (Phila). 2010;3(6):696-706. PMCID: 2935331.
48. Gail MH, Costantino JP, Bryant J, Croyle R, Freedman L, Helzlsouer K, et al. Weighing the risks and benefits of tamoxifen treatment for preventing breast cancer. J Natl Cancer Inst. 1999;91(21):1829-46.
49. Physicians’ Desk Reference. Evista. Physicians’ Desk Reference. Montvael, NJ: Thompson; 2005. p. 1836-9.
50. Kanis JA, Borgstrom F, Johnell O, Oden A, Sykes D, Jonsson B. Cost-effectiveness of raloxifene in the UK: an economic evaluation based on the MORE study. Osteoporos Int. 2005;16(1):15-25.
51. Mobley LR, Hoerger TJ, Wittenborn JS, Galuska DA, Rao JK. Cost-effectiveness of osteoporosis screening and treatment with hormone replacement therapy, raloxifene, or alendronate. Med Decis Making. 2006;26(2):194-206.
52. Stevenson M, Lloyd Jones M, De Nigris E, Brewer N, Davis S, Oakley J. A systematic review and economic evaluation of alendronate, etidronate, risedronate, raloxifene and teriparatide for the prevention and treatment of postmenopausal osteoporosis. Health Technol Assess. 2005;9(22):1-160.
53. Chen WY, Rosner B, Colditz GA. Moving forward with breast cancer prevention. Cancer. 2007;109(12):2387-91.
54. Armstrong B, Doll R. Environmental factors and cancer incidence and mortality in different countries, with special reference to dietary practices. Int J Cancer. 1975;15:617-31.
55. Howe GR, Hirohata T, Hislop TG, Iscovich JM, Yuan JM, Katsouyanni K, et al. Dietary factors and risk of breast cancer: combined analysis of 12 case-control studies. J Natl Cancer Inst. 1990;82:561-9.
56. Giovannucci E, Stampfer MJ, Colditz GA, Manson JE, Rosner BA, Longnecker M, et al. A comparison of prospective and retrospective assessments of diet in the study of breast cancer. Am J Epidemiol. 1993;137:502-11.
57. Hunter DJ, Spiegelman D, Adami HO, Beeson L, van den Brandt PA, Folsom AR, et al. Cohort studies of fat intake and the risk of breast cancer: a pooled analysis. N Eng J Med. 1996;334:356-61.
58. Prentice RL, Caan B, Chlebowski RT, Patterson R, Kuller LH, Ockene JK, et al. Low-fat dietary pattern and risk of invasive breast cancer: the Women’s Health Initiative Randomized Controlled Dietary Modification Trial. JAMA. 2006;295(6):629-42.
59. Willett WC. Specific fatty acids and risks of breast and prostate cancer: dietary intake. Am J Clin Nutr. 1997;66 (suppl):1557s-63s.
60. Cohen LA, Kendall ME, Zang E, Meschter C, Rose DP. Modulation of N-nitrosomethylurea-induced mammary tumor promotion by dietary fiber and fat. J Natl Cancer Inst. 1991;83:496-501.
61. Martin-Moreno JM, Willett WC, Gorgojo L, Banegas JR, Rodriguez-Artalejo F, Fernandez-Rodriguez JC, et al. Dietary fat, olive oil intake and breast cancer risk. Int J Cancer. 1994;58:774-80.
62. Cho E, Spiegelman D, Hunter DJ, Chen WY, Stampfer MJ, Colditz GA, et al. Premenopausal fat intake and risk of breast cancer. J Natl Cancer Inst. 2003;95(14):1079-85.
63. Goldin BR, Aldercreutz H, Gorbach SL, Warram JH, Dwyer JT, Swenson L, et al. Estrogen excretion patterns and plasma levels in vegetarian and omnivorous women. N Engl J Med. 1982;307:1542-7.
64. Willett WC, Hunter DJ, Stampfer MJ, Colditz GA, Manson JE, Spiegelman D, et al. Dietary fat and fiber in relation to risk of breast cancer: An 8-year follow-up. J Am Med Assoc. 1992;268:2037-44.
65. Verhoeven DTH, Assen N, Goldbohm RA, Dorant E, van’t Veet P, Sturmans F, et al. Vitamins C and E, retinol, beta-carotene and dietary fiber in relation to breast cancer risk: a prospective cohort study. Br J Cancer. 1997;75:149-55.
66. Rohan TE, Howe GR, Friedenreich CM, Jain M, Miller AB. Dietary fiber, vitamins A, C, and E, and risk of breast cancer: a cohort study. Cancer Causes Control. 1993;4:29-37.
67. World Cancer Research Fund, American Institute for Cancer Research. Food, Nutrition and the Prevention of Cancer: a Global Perspective. Washington, DC: American Institutue for Cancer Research; 1997.
68. Dorgan JF, Sowell A, Swanson CA, Potischman N, Miller R, Schussler N, et al. Relationships of serum carotenoids, retinol, a-tocopherol, and selenium with breast cancer risk: results from a prospective study in Columbia, Missouri (United States). Cancer Causes Control. 1998;9:89-97.
69. Tamimi RM, Hankinson SE, Campos H, Spiegelman D, Zhang S, Colditz GA, et al. Plasma carotenoids, retinol, and tocopherols and risk of breast cancer. Am J Epidemiol. 2005;161(2):153-60.
70. Willett WC. Diet and breast cancer. J Intern Med. 2001;249:395-411.
71. Freudenheim JL, Ambrosone CB, Moysich KB, Vena JE, Graham S, Marshall JR, et al. Alcohol dehydrogenase 3 genotype modification of the association of alcohol consumption with breast cancer risk. Cancer Causes and Control. 1999;10:369-77.
72. Zhang S, Hunter DJ, Hankinson SE, Giovannucci EL, Rosner BA, Colditz GA, et al. A prospective study of folate intake and the risk of breast cancer. JAMA. 1999;281:1632-7.
73. Zhang SM, Willett WC, Selhub J, Hunter DJ, Giovannucci EL, Holmes MD, et al. Plasma folate, vitamin B6, vitamin B12, and homocysteine and risk of breast cancer. JNCI. 2003;95:373-80.
74. Smith-Warner SA, Spiegelman D, Yaun SS, Adami HO, Beeson WL, van den Brandt PA, et al. Intake of fruits and vegetables and risk of breast cancer: a pooled analysis of cohort studies. JAMA. 2001;285:769-76.
75. Smith-Warner SA, Spiegelman D, Yaun S-S, Adami HO, van den Brandt PA, Folsom AR, et al. Alcohol and breast cancer in women: a pooled analysis of cohort studies. J Am Med Assoc. 1998;279:535-40.
76. Longnecker MP, Newcomb PA, Mittendorf R, Greenberg ER, Clapp RW, Bogdan GF, et al. Risk of breast cancer in relation to lifetime alcohol consumption. J Natl Cancer Inst. 1995;87:923-9.
77. Reichman ME, Judd JT, Longcope C, Schatzkin A, Clevidence BA, Nair PP, et al. Effects of alcohol consumption on plasma and urinary hormone concentrations in premenopausal women. J Natl Cancer Inst. 1993;85:722-7.
78. Dorgan JF, Baer DJ, Albert PS, Judd JT, Brown ED, Corle DK, et al. Serum hormones and the alcohol-breast cancer association in postmenopausal women. J Natl Cancer Inst. 2001;93:710-5.
79. Ginsburg ES, Walsh BW, Gao XP, Gleason RE, Feltmate C, Barbieri RL. The effect of acute ethanol ingestion on estrogen levels in postmenopausal women using transdermal estradiol. J Soc Gynecol Invest. 1995;2:26-9.
80. Sellers TA, Kushi LH, Cerhan JR, Vierkant RA, Gapstur SM, Vachon CM, et al. Dietary folate intake, alcohol, and risk of breast cancer in a prospective study of postmenopausal women. Epidemiology. 2001;12:420-8.
81. Rimm E, Willett W, Manson J, Speizer F, Hennekens C, Stampfer M. Folate and vitamin B6 intake and risk of myocardial infarction among US women (abstract). Am J Epidemiol. 1996;143(suppl):S36.
82. Giovannucci E, Stampfer MJ, Colditz GA, Hunter DJ, Fuchs C, Rosner BA, et al. Multivitamin use, folate, and colon cancer in women in the Nurses’ Health Study. Ann Intern Med. 1998;129:517-24.
83. Petrakis N, Barnes S, King E, Lowenstein J, Wienke J, Lee M, et al. Stimulatory influence of soy protein on breast secretion inpre-and postmenopausal women. Cancer Epidemiol Biomarkers Prev. 1996;5:785-94.
84. McMichael-Phillips D, harding C, Morton M, Roberts S, Howell A, Potten C, et al. Effects of soy-protein supplementation on epithelial proliferation in the histologically normal human breast. Am J Clin Nutr. 1998;68:1431S-5S.
85. Hargreaves D, Potten C, Harding C, Shaw L, Morton M, Roberts S, et al. Two-week dietary soy supplementation has an estrogenic effect on normal premenopausal breast. J Clin Endocrinol Metab. 1999;84:4017-24.
86. Key T, Sharp G, Appleby P, Beral V, Goodman M, Soda M, et al. Soya foods and breast cancer risk: a prospective study in Hiroshima and Nagasaki, Japan. Br J Cancer. 1999;81:1248-56.
87. Hooper L, Ryder JJ, Kurzer MS, Lampe JW, Messina MJ, Phipps WR, et al. Effects of soy protein and isoflavones on circulating hormone concentrations in pre- and post-menopausal women: a systematic review and meta-analysis. Hum Reprod Update. 2009;15(4):423-40. PMCID: 2691652.
88. Kurzer MS. Soy consumption for reduction of menopausal symptoms. Inflammopharmacology. 2008;16(5):227-9.
89. van Ee JH. Soy constituents: modes of action in low-density lipoprotein management. Nutr Rev. 2009;67(4):222-34.
90. Carlson S, Peng N, Prasain JK, Wyss JM. Effects of botanical dietary supplements on cardiovascular, cognitive, and metabolic function in males and females. Gend Med. 2008;5 Suppl A:S76-90. PMCID: 2675052.
91. Wu AH, Yu MC, Tseng CC, Pike MC. Epidemiology of soy exposures and breast cancer risk. Br J Cancer. 2008;98(1):9-14. PMCID: 2359677.
92. World Cancer Research Fund. Food, Nutrition, Physical Activity, and the Prevention of Cancer: a Global Perspective. Washington, DC: AICR; 2007.
93. Goodwin PJ, Ennis M, Pritchard KI, Koo J, Hood N. Prognostic effects of 25-hydroxyvitamin D levels in early breast cancer. J Clin Oncol. 2009;27(23):3757-63.
94. Jacobs ET, Thomson CA, Flatt SW, Al-Delaimy WK, Hibler EA, Jones LA, et al. Vitamin D and breast cancer recurrence in the Women’s Healthy Eating and Living (WHEL) Study. Am J Clin Nutr. 2010.
95. van den Brandt PA, Spiegelman D, Yaun SS, Adami HO, Beeson L, Folson AR, et al. Pooled analysis of prospective cohort studies on height, weight, and breast cancer risk. Am J Epidemiol. 2000;152:514-27.
96. Hunter DJ, Willett WC. Diet, body size, and breast cancer. Epidemiol Rev. 1993;15:110-32.
97. Ursin G, Longnecker MP, Halies RW, Greenland S. A meta-analysis of body mass index and risk of premenopausal breast cancer epidemiology. Epidemiology. 1995;6:137-41.
98. Rich-Edwards JW, Goldman MB, Willett WC, Hunter DJ, Stampfer MJ, Colditz GA, et al. Adolescent body mass index and ovulatory infertility. Am J Obstet Gynecol. 1994;171:171-7.
99. Ziegler RG, Hoover RN, Nomura AMY, West DW, Wu AH, Pike MC, et al. Relative weight, weight change, height, and breast cancer risk in Asian-American women. J Natl Cancer Inst. 1996;88:650-60.
100. Barnes-Josiah D, Potter JD, Sellers TA, Himes JH. Early body size and subsequent weight gain as predictors of breast cancer incidence (Iowa, United States). Cancer Causes Control. 1995;6:112-8.
101. Huang Z, Hankinson SE, Colditz GA, Stampfer MJ, Hunter DJ, Manson JE, et al. Dual effects of weight and weight gain on breast cancer risk. J Am Med Assoc. 1997;278:1407-11.
102. Le Marchand L, Kolonel LN, Earle ME, Mi MP. Body size at different periods of life and breast cancer risk. Am J Epidemiol. 1988;128:137-52.
103. Eliassen AH, Colditz G, Rosner B, Willett W, Hankinson SE. Adult weight change and risk of postmenopausal breast cancer. JAMA. 2006;296:193-201.
104. International Agency for Research on Cancer. Weight Control and Physical Activity. Lyon: International Agency for Research on Cancer; 2002.
105. Bernstein L, Patel AV, Ursin G, Sullivan-Halley J, Press MF, Deapen D, et al. Lifetime recreational exercise activity and breast cancer risk among black women and white women. J Natl Cancer Inst. 2005;97(22):1671-9.
106. Bernstein L, Henderson BE, Hanisch R, Sullivan-Halley J, Ross RK. Physical exercise and reduced risk of breast cancer in young women. J Natl Cancer Inst. 1994;86:1403-8.
107. Maruti SS, Willett WC, Feskanich D, Rosner B, Colditz GA. A Prospective Study of Age-Specific Physical Activity and Premenopausal Breast Cancer. J Natl Cancer Inst. 2008.
108. Kelsey JL, Gammon MD, John EM. Reproductive factors and breast cancer. Epidemiol Rev. 1993; 15:36-47.
109. Bruzzi P, Negri E, La Vecchia C. Short term increase in risk of breast cancer after full term pregnancy. Br Med J. 1988;297:1096-8.
110. Pike MC, Krailo MD, Henderson BE, Casagrande JT, Hoel DG. “Hormonal” risk factors, “breast tissue age” and the age-incidence of breast cancer. Nature. 1983;303:767-70.
111. Rosner B, Colditz GA, Willett WC. Reproductive risk factors in a prospective study of breast cancer: the Nurses’ Health Study. Am J Epidemiol. 1994;139:819-35.
112. Rosner B, Colditz G. Extended mathematical model of breast cancer incidence in the Nurses’ Health Study. J Natl Cancer Inst. 1996;88:359-64.
113. Trichopoulos D, Hsieh C, MacMahon B, Lin T, Lowe C, Mirra A. Age at any birth and breast cancer risk. Int J Cancer. 1983;31:701-4.
114. Lane-Claypon JE. A further report on cancer of the breast, with special reference to its associated antecedent conditions. London: Ministry of Health; 1926.
115. Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and breastfeeding: collaborative reanalysis of individual data from 47 epidemiological studies in 30 countries, including 50302 women with breast cancer and 96973 women without the disease. Lancet. 2002;360(9328):187-95.
116. Melbye M, Wohlfahrt J, Olsen J. Induced abortion and the risk of breast cancer. N Engl J Med. 1997;336:81-5.
117. Trichopoulos D, MacMahon B, Cole P. Menopause and breast cancer risk. J Natl Cancer Inst. 1972;48:605-13.
118. Lilienfeld AM. The relationship of cancer of the female breast to artificial menopause and marital status. Cancer. 1956;9:927-34.
119. Colditz G, Rosner B. Cumulative risk of breast cancer to age 70 years according to risk factor status: data from the Nurses’ Health Study. Am J Epidemiol. 2000;152(10):950-64.
120. Dupont WD, Page DL. Risk factors for breast cancer in women with proliferative breast disease. N Engl J Med. 1985;312:146-51.
121. Tamimi RM, Byrne C, Baer HJ, Rosner B, Schnitt SJ, Connolly JL, et al. Benign breast disease, recent alcohol consumption, and risk of breast cancer: a nested case-control study. Breast Cancer Res. 2005;7(4):R555-62.
122. Marshall LM, Hunter DJ, Connolly JL, Schnitt SJ, Byrne C, London SJ, et al. Risk of breast cancer associated with atypical huperplasia of lobular and ductal types. Cancer Epidemiol Biomarkers Prev. 1997;6:297-301.
123. Hartmann LC, Sellers TA, Frost MH, Lingle WL, Degnim AC, Ghosh K, et al. Benign breast disease and the risk of breast cancer. N Engl J Med. 2005;353(3):229-37.
124. Colditz G, Rosner B, Chen WY, Holmes M, Hankinson SE. Risk factors for breast cancer:according to estrogen and progesterone receptor status. J Natl Cancer Inst. 2004;96:218-28.
125. Adami HO, Signorello LB, Trichopoulos D. Towards an understanding of breast cancer etiology. Semin Cancer Biol. 1998;8(4):255-62.
126. Trichopoulos D. Is breast cancer initiated in utero? Epidemiology. 1990;1:95-6.
127a. Potischman N, Troisi R. In-utero and early life exposures in relation to risk of breast cancer. Cancer Causes Control. 1999;10(6):561-73.
127b. Xue F and Michels K. Intrauterine factors and risk of breast cancer: a systematic review and meta-analysis of current evidence. Lancet Oncology. 2007; 8(12):1088-1100.
127c. Michels K and Xue F. Role of birthweight in the etiology of breast cancer. Int J Cancer. 2006;119(9):2007-25.
128. Freudenheim JL, Marshall JR, Graham S, Laughlin R, Vena JE, Bandera E, et al. Exposure to breastmilk in infancy and the risk of breast cancer. Epidemiology. 1994;5(3):324-31.
129. Colditz G, Frazier A. Models of breast cancer show that risk is set by events of early life: prevention efforts must shift focus. Cancer Epidemiol Biomarkers Prev. 1995;4:567-71.
130. Russo J, Gusterson BA, Rogers AE, Russo IH, Wellings SR, van Zwieten MJ. Biology of disease: comparison study of human and rat mammary tumorigenesis. Lab Invest. 1990;62:244-78.
131. Tokunaga M, Land C, Tokuoka S, Nishimori I, Soda M, Akiba S. Incidence of female breast cancer among atomic bomb survivors 1950-1985. Radiat Res. 1994;138:209-23.
132. Miller AB, Howe GR, Sherman GJ, Lindsay JP, Yaffe MJ, Dinner PJ, et al. Mortality from breast cancer after irradiation during fluoroscopic examinations in patients being treated for tuberculosis. N Eng J Med. 1989;321:1285-9.
133. Hancock S, Tucker M, Hoppe R. Breast cancer after treatment of Hodgkin’s disease. J Natl Cancer Inst. 1993;85:25-31.
134. Berkey CS, Frazier AL, Gardner JD, Colditz GA. Adolescence and breast carcinoma risk. Cancer. 1999;85(11):2400-9.
135. Baer HJ, Colditz GA, Rosner B, Michels KB, Rich-Edwards JW, Hunter DJ, et al. Body fatness during childhood and adolescence and incidence of breast cancer in premenopausal women: a prospective cohort study. Breast Cancer Res. 2005;7(3):R314-25.
136. Baer HJ, Schnitt SJ, Connolly JL, Byrne C, Willett WC, Rosner B, et al. Early life factors and incidence of proliferative benign breast disease. Cancer Epidemiol Biomarkers Prev. 2005;14(12):2889-97.
137. Frazier AL, Ryan CT, Rockett H, Willett WC, Colditz GA. Adolescent diet and risk of breast cancer. Breast Cancer Res. 2003;5(3):R59-64.
138. Baer H, Schnitt SJ, Connolly JL, Byrne C, Cho E, Willett WC, et al. Adolescent Diet and Incidence of Benign Breast Disease. J Natl Cancer Inst. 2003;Submitted publication.
139. Frazier AL, Li L, Cho E, Willett WC, GA C. Adolescent Diet and Risk of Breast Cancer (United States). Cancer Causes and Control. 2004;15:73-82.
140. Boyd NF, Byng JW, Jong RA, Fishell KK, Little LE, Miller AB, et al. Quantitative classification of mammographic densities and breast cancer risk: results from the Canadian National Breast Screening Trial. J Natl Cancer Inst. 1995;87:670-5.
141. Byrne C, Schairer C, Wolfe J, Parekh N, Salane M, Brinton LA, et al. Mammographic features and breast cancer risk: effects with time, age, and menopause status. J Natl Cancer Inst. 1995;87(21):1622-9.
142. Spicer DV, Ursin G, Parisky YR, Pearce JG, Shoupe D, Pike A, et al. Changes in mammographic densities induced by a hormonal contraceptive designed to reduce breast cancer risk. J Natl Cancer Inst. 1994;86(6):431-6.
143. Tamimi RM, Hankinson SE, Colditz GA, Byrne C. Endogenous sex hormone levels and mammographic density among postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2005;14(11 Pt 1):2641-7.
144. Tamimi RM, Byrne C, Colditz GA, Hankinson SE. Endogenous hormone levels, mammographic density, and subsequent risk of breast cancer in postmenopausal women. J Natl Cancer Inst. 2007;99(15):1178-87.
145. Laden F, Collman G, Iwamoto K, Alberg AJ, Berkowitz GS, Freudenheim JL, et al. 1,1-Dichloro-2,2-bis(p-chlorophenyl)ethylene and polychlorinated biphenyls and breast cancer: combined analysis of five U.S. studies. J Natl Cancer Inst. 2001;93(10):768-76.
146. Mirick DK, Davis S, Thomas DB. Antiperspirant use and the risk of breast cancer. J Natl Cancer Inst. 2002;94(20):1578-80.
147. Brinton LA, Brown SL. Breast implants and cancer. J Natl Cancer Inst. 1997;89:1341-9.
148. Deapen DM, Bernstein L, Brody GS. Are breast implants anticarcinogenic? A 14-year follow-up of the Los Angeles Study. Plast Reconstr Surg. 1997;99:1346-53.
149. Brinton LA, Malone KE, Coates RJ, Schoenberg JB, Swanson CA, Dalingh JR, et al. Breast enlargement and reduction: results from a breast cancer case-control study. Plast Reconstr Surg. 1996;97:269-75.
150. Bryant H, Brasher P. Breast implants and breast cancer – reanalysis of a linkage study. New England Journal of Medicine. 1995;332:1535-9.
151. Wald N, Hackshaw A, Frost C. When can a risk factor be used as a worthwhile screening test? BMJ. 1999;319:1562-5.
152. Jacobs TW, Byrne C, Colditz G, Connolly JL, Schnitt SJ. Radial scars in benign breast biopsy specimens and breast cancer risk: a case-control study. NEJM. 1999;340:430-6.
153. Endogenous Hormones and Breast Cancer Collaborative Group. Endogenous sex hormones and breast cancer in postmenopausal women: reanalysis of nine prospective studies. J Natl Cancer Inst. 2002;94(8):606-16.
154. Claus EB, Risch N, Thompson WD. The calculation of breast cancer risk for women with a first degree family history of ovarian cancer. Breast Cancer Res Treat. 1993;28(2):115-20.
155. Berry DA, Iversen ES, Jr., Gudbjartsson DF, Hiller EH, Garber JE, Peshkin BN, et al. BRCAPRO validation, sensitivity of genetic testing of BRCA1/BRCA2, and prevalence of other breast cancer susceptibility genes. J Clin Oncol. 2002;20(11):2701-12.
156. Parmigiani G, Berry D, Aguilar O. Determining carrier probabilities for breast cancer-susceptibility genes BRCA1 and BRCA2. Am J Hum Genet. 1998;62(1):145-58.
157. Tyrer J, Duffy SW, Cuzick J. A breast cancer prediction model incorporating familial and personal risk factors. Stat Med. 2004;23(7):1111-30.
158. Rockhill B, Byrne C, Rosner B, Louie MM, Colditz G. Breast cancer risk prediction with a log-incidence model: evaluation of accuracy. J Clin Epidemiol. 2003;56(9):856-61.
159. Rosner B, Colditz GA, Iglehart JD, Hankinson SE. Risk prediction models with incomplete data with application to prediction of estrogen receptor-positive breast cancer: prospective data from the Nurses’ Health Study. Breast Cancer Res. 2008;10(4):R55.
160. Tice JA, Cummings SR, Ziv E, Kerlikowske K. Mammographic breast density and the Gail model for breast cancer risk prediction in a screening population. Breast Cancer Res Treat. 2005;94(2):115-22.