2008-08-01
A pacemaker is a mechanical device that in most cases prevents the heart from beating too slowly or from stopping entirely.
I. Normal Heart Function
In
order to understand pacemakers, it is first important to become
familiar with the basic anatomy and physiology (or function) of the
heart. The heart has four chambers. The top two chambers, called the atria, are relatively small and thin. The bottom two chambers, the ventricles,
are larger and thicker. Blood that has been drained from all of the
body’s tissues (except for the lungs) flows through the veins into the
right atrium. The right atrium then contracts, propelling blood across the tricuspid valve and into the right ventricle. The right ventricle then squeezes that blood across the pulmonary valve, through the pulmonary artery and into the lungs. Once in the lungs, the blood is re-supplied with oxygen and continues on its way via the pulmonary veins into the left atrium. The left atrium then contracts, pushing the blood across the mitral valve and into the left ventricle. Finally, the left ventricle squeezes the blood across the aortic valve, through a large artery called the aorta and on to perfuse the tissues of the body.
The normal electrical conduction system of the heart
The
right and the left sides of the heart actually work simultaneously, so
that the atria both contract together, and then the ventricles contract
together. These coordinated pumping actions are orchestrated by the
heart’s electrical conduction system, and the sinus node
can be thought of as the conductor of that orchestra. The sinus node is
the normal or physiologic pacemaker. From the sinus node, situated in
the upper right atrium, the electrical signal responsible for each heart
beat arises. Normally, in response to signals from the brain or the
adrenal glands, the sinus node will speed up as needed (such as during
exercise, anxiety, or excitement) and slow down when appropriate (such
as during rest or sleep).
How
does that sinus impulse activate the heart? It is important to
understand that the muscle cells of the heart (the same cells
responsible for contracting and relaxing the chambers) can conduct
electricity from one cell to the next. For example, an electrical signal
that starts in one part of the right atria can travel from cell to cell
throughout the right atrium and into the left atrium (the right and
left atrium are immediately connected and share the same septum [a thin
dividing wall]). Similarly, an electrical impulse that starts in one
ventricle can propagate from one ventricle to the other. Normally, the
atria are separated from the ventricles by a fibrous structure that
houses the tricuspid and mitral valves; in essence, this structure
electrically disconnects the two atria from the two ventricles.
Consequently, in a normal heart, there is only one way for an electrical
signal in the atria to communicate down to the ventricles, and that is
via the AV node. The AV node is situated in the middle of the
heart, receiving electrical connections from the atria and delivering
electrical connections to the ventricles.
When
a heart muscle cell is activated by an electrical signal, it contracts.
In sum, a normal heart beat arises in the sinus node and propagates
across the right atrium and the left atrium via cell to cell electrical
connections, resulting in contraction of both atria nearly at the same
time. When it reaches the lower atrial septum, it then continues to the
AV node. After traveling through the AV node, this electrical signal
continues down a network of specialized conduction tissue that spreads
the impulse in an organized fashion throughout the ventricles, resulting
in an organized contraction of each ventricle nearly simultaneously
(Figure 1). The first part of this specialized conduction is the bundle of His, and then the conduction system splits into the right bundle branch (supplying the right ventricle) and the left bundle branch (supplying the left ventricle).
Figure 1.
The normal conduction system of the heart. Yellow arrows demonstrate an
electrical signal originating in the sinus node and propagating through
both the right and left atria. This signal will reach the AV node and
travel through the ventricular conduction system (shown in gold),
ultimately electrically activating the right and left ventricles. The
left atrial appendage, a potential site of blood clot formation in
atrial fibrillation, is also shown. This figure was obtained with
permission from Mr. David Criley at www.blaufuss.org.
II. What are the indications for a pacemaker?
With
the exception of biventricular pacemakers (discussed below in Section
VII), the general purpose of a pacemaker is to prevent the heart from
going too slow. Therefore, in very general terms, a pacemaker is
indicated when the heart is either going too slow or exhibits evidence
that it is at significant risk of going too slow.1
“Too
slow” might seem to imply a certain number or a certain number of heart
beats per minute, but it is actually a bit more complex than that.
While the normal heart rate or pulse (or, the number of times the sinus
node sends a signal) is generally between 60 and 100 beats per minute,
the true normal range can vary and depends largely on activity. For
example, it would not be abnormal for an elite athlete to have a heart
rate above 150 beats per minute during heavy exertion. Also, healthy
people may drop their heart rates into the 30’s while asleep. So, rather
than a particular number of beats per minute, “too slow” essentially
means slow enough that it is adversely affecting a person’s life.
When
the heart rate is too slow, an insufficient amount of blood is ejected
forward by the heart. This can result in a number of symptoms: if the
problem is primarily that the heart rate can not increase sufficiently
during exertion, the main symptom may be fatigue and perhaps shortness
of breath. If there is a significant loss of forward blood flow, a
person may experience chest pain or pressure (those with obstructions in
the blood vessels that supply blood to the heart, or coronary artery disease,
may be particularly prone to chest pain or pressure). In those in whom
the pulse is dangerously slow, or, more commonly, in those in whom the
pulse rate suddenly drops by a substantial amount, the primary symptom
may be a sensation that they are about to faint (called presyncope) or a true faint (a sudden loss of consciousness called syncope).
Syncope can be particularly dangerous as it may result in injury,
particularly when doing things such as walking up or down stairs,
crossing the street, or driving.
Usually,
an assessment of the heart rate alone is insufficient. Physicians
generally will obtain an electrocardiogram (also called an ECG or an
EKG), which involves having several electrodes placed on the body. It is
painless and typically takes less than five minutes. With this test,
the electrical activity of the heart can be seen: a small wave, called
the P wave, corresponds to electrical activation of the atrium during normal sinus rhythm. In a normal rhythm, the larger QRS complex,
representing electrical activation of the ventricles, follows shortly
after the P wave is inscribed (Figure 2). A person may wear a portable
EKG monitor at home or while staying in the hospital in order to monitor
the heart rhythm over a long period of time. With this monitoring, the
nature of the heart rhythm can be understood. It is important to
understand that, sometimes, a patient may not have clear symptoms of an
inappropriately slow heart rate, but that monitoring may reveal evidence
of impending dangerous block in the conduction system or a dangerously
slow heart rate. Therefore, at times, a cardiologist or cardiac
electrophysiologist may deem it necessary to place a pacemaker even when
symptoms are not apparent.
Figure 2.
The electrocardiographic (also called EKG or ECG) recording
demonstrates normal sinus rhythm, with deflections called P waves
(denoted by asterisks) that represent normally conducting atria. Each P
wave is followed by a QRS complex, representing ventricular
depolarization (solid arrows). Each QRS complex is followed by a T wave,
representing repolarization of the ventricles (dashed arrows).
In
order to assess if the heart rate is inappropriately slow with
activity, a person may undergo an exercise treadmill test, during which
the heart rate and rhythm can be assessed with a measurable amount of
exertion. If the heart rate remains inappropriately slow with activity,
the patient is said to have chronotropic incompetence, and a pacemaker may be indicated.
What causes a heart to be too slow?
Typically,
the problem can be found in either the sinus node, the AV node, or the
conduction system that connects the AV node to the ventricles (see
Section I. for normal function of these structures). While there are
many possible reasons for electrical conduction slowing or block in the
heart, the most common reasons are fibrosis (or scarring) related to
age, a loss of blood flow to the conduction system, or medicines.2 Most commonly, the reason for dysfunction is related to age: in some people, a build up of fibrosis,
or scar tissue, occurs in these structures, leading to either slowing
of electrical conduction or complete loss of electrical conduction. Less
commonly, a loss of blood flow to one of these structures can result in
conduction slowing or block- such a loss of blood flow would most
commonly occur when there is an abrupt occlusion (or blockage) of the
blood vessels supplying blood (and, with it, oxygen) to the heart as in
the case of a heart attack (also called a myocardial infarction).
Another common cause or contributing factor can be medicines that might
slow or block conduction through a variety of mechanisms. Sometimes,
this can be a reversible cause, and discontinuing the medicine can be an
adequate solution. Other times, such a medicine may be necessary, or
the mechanism by which the medicine resulted in electrical conduction
problems is permanent.
Sinus node problems
If
the slowing occurs in the sinus node, the problem is the generation of
impulses that will result in a heart beat. Fortunately, as other parts
of the heart can generate their own impulse (albeit at a slower rate),
this does not usually result in drastic consequences. Therefore,
usually, if the sinus node does not adequately generate or propagate an
electrical impulse, an escape rhythm will arise and take over: in
this case, a different part of the heart that can autonomously generate
an electrical impulse (albeit typically at a rate slower that the
normal sinus rate) will arise as the new source of electric signals.
However, the presence of such an escape rhythm can not be guaranteed,
and, when present, it may be so slow as to result in significant
symptoms (consistent with those described above in this Section). If the
sinus node abruptly stops, there may not be sufficient time for an
escape rhythm to kick-in before a substantial loss of heart output (or cardiac output) causes a person to lose consciousness (Figure 3). Of note, when a person has chronotropic incompetence
(described above as an inability to mount adequate heart rates with
activity), it is due to a problem with the sinus node. In fact, the most
common indication for a pacemaker is a problem with the sinus node
(sometimes call sick sinus syndrome).
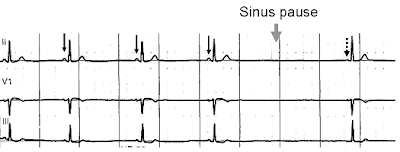
Figure 3.
Normal sinus rhythm, with solid black arrows pointing to normal P waves
representative of normal sinus node function, followed by a pause in
sinus node activity (resulting in a transient loss of heart beats). Note
that the P wave that disrupts the pause (indicated by the dashed arrow)
does not look like the previous (normal) P waves- this last P wave is
arising from a different part of the atrium, representing an escape rhythm.
Tachy-brady syndrome
One
specific circumstance of sinus node problems that is a bit more complex
and in fact quite common involves a conundrum that can develop when a
person has both rhythms that are too fast at times and too slow at other
times. Most commonly, this occurs in the setting of a common fast
rhythm that can affect the atria called atrial fibrillation: briefly,
this is a rhythm characterized by rapid chaotic activity in the atria
that propagates down to the ventricles, resulting in a rapid and
irregular pulse (see separate Google knol entitled Atrial Fibrillation). This rhythm can be intermittent (so called paroxysmal atrial fibrillation),
and the treatment often involves medicines that slow the AV node in
order to prevent the ventricles from going inappropriately fast.
However, as these medicines also slow the sinus node when atrial
fibrillation is not present, a side effect of all these medicines is
that the pulse can become too slow. Therefore, these patients may go
very fast during atrial fibrillation, requiring high doses of AV nodal
blockers (such as calcium channel blockers or beta-blockers) to prevent
the pulse from going to fast, but, when in sinus rhythm, those same
agents (which also slow conduction of the sinus node) can result in too
slow of a heart rate. Such patients are also often deemed as having tachy-brady syndrome:
“tachycardia” means a fast heart rate and “bradycardia” means a slow
heart rate, and these patients exhibit both. When this is an issue, the
treatment is to place a permanent pacemaker, which prevents the heart
from going too slow. Then, medicines can be given to prevent the heart
from going too fast, and the patient remains protected against
bradycardia.
Problems with the AV junction
The AV junction can be another site of conduction slowing or block requiring a pacemaker. The AV junction essentially
includes the AV node and the conduction tissue just below the AV node:
this would include the bundle of His as well as potentially both bundle
branches (as described in Section I). Not uncommonly, one of the bundle
branches can be significantly slowed or blocked, but this usually does
not cause a problem: for example, if the right bundle branch can not
conduct an electrical impulse, the impulse will travel from the AV node,
to the bundle of His and down the left bundle branch. From there, the
right ventricle will be depolarized from heart muscle cell to heart
muscle cell. Although such conduction is not as fast and efficient as
traveling down the normal conduction system, it is sufficient to
activate the ventricle. Therefore either a right or left bundle branch
block by itself (assuming all else is well) is not an indication for a
pacemaker. However, when one bundle branch is blocked, the heart is not
electrically activated in as fast or coordinated a fashion as it would
be if the specialized conduction system were intact. In addition, the
pumping action of the heart may be affected in some people, delaying
activation and contraction, a condition known as mechanical dyssynchrony
(discussed below, Section VII).
Importantly,
as noted above, the atria are not connected to the ventricles
electrically; except in rare circumstances, no heart muscle cells bridge
the divide occupied by the electrically silent heart valves. Therefore,
if the AV node, bundle of His, or both bundle branches are blocked,
electrical signals can not reach the ventricles. In this situation, AV
block is present. As with sinus node conduction problems, AV slowing or
AV block can be intermittent or persistent. If there is complete AV
block, a more slowly activated focal escape rhythm may arise,
allowing the ventricles to continue to beat, albeit usually at a much
slower rate (Figure 4). In some circumstances, an escape rhythm either
is not present or takes so much time to develop that a patient may lose
consciousness due to lack of a heart beat. Depending on the nature of
the escape rhythm, the severity of the AV slowing or block, and whether
the condition is persistent or intermittent, AV conduction problems
essentially result in any or all of the same symptoms discussed above:
fatigue, shortness of breath, chest discomfort, feeling faint (presyncope) or a loss of consciousness (syncope).
In addition, in certain patients deemed to be at high risk based on
their medical history, there are a number of different types of AV block
that can be detected by electrocardiographic monitoring, and some types
may portend such a high risk for dangerous conduction problems that a
pacemaker can be indicated even in the absence of the traditional
symptoms mentioned here. Again, as with sinus node problems, either a
cardiologist or a cardiologist with a specialty in heart rhythm
disturbances (a cardiac electrophysiologist, also called an EP for short) is typically consulted as an expert in determining if a pacemaker is indicated in any one particular case.
III. How is a pacemaker placed in the body?
The set-up
A pacemaker will typically be put in by a heart surgeon, cardiologist, or cardiac electrophysiologist in an operating room, cardiac catheterization laboratory (the “cath lab”), or an electrophysiology laboratory
(the “EP Lab”). Regardless, the room will be sterile in order to
minimize the risk of infection, staffed by nurses and sometimes
technicians, with fluoroscopy (or X-ray equipment) available to
be positioned over the operating table. The representative from the
company manufacturer of the device may
be present.
be present.
Patients
coming in for this procedure will usually be asked to fast after
midnight prior to the day of the procedure (medicines with small sips of
water are typically acceptable). Prior to beginning the procedure, an
intravenous (or “IV” line) is first placed in the patient and,
typically, sedating and relaxing medicines are administered through the
IV line. Prophylactic (or preventive) antibiotics are also usually
administered in order to minimize the risk of infection. The area of the
chest will be cleaned with a solution designed to kill bacteria on the
skin, and a sterile drape will be placed over the patient’s body.
Typically, pacemakers are placed in the left chest, but, if the patient
is left handed or performs activities that might make a left-sided
placement suboptimal, it is placed on the right. Of note, the
description of this procedure relates to the majority of patients
undergoing pacemaker placement, namely adults without significant
structural heart disease or vascular disease. The nature of the
procedure may be quite different in infants and young children or people
with either long-standing pacemaker leads already in place or unusual
vascular or heart anatomy.
The pocket
Local
anesthetic is given with a small needle in the area where the pacemaker
will be placed. This typically burns when it is first injected, but
very quickly becomes numb. Then, the initial incision is made. This is
typically approximately 3-6 cm long and can be made just under the
clavicle (collar bone) or at a diagonal, parallel to the groove between
the shoulder muscle and the chest. Of note, as with many surgical
procedures, there are subtle differences in technique between operators,
and this review is meant to describe the basic concepts of the
procedure. After the incision is made, a small “pocket” is then made
just under the skin. In some circumstances, the pocket may be made a bit
deeper, under the muscle- while a decision to make the pocket
“sub-pectoral” (or under the muscle) may be the general preference of
the operator, it is typically used with a goal of minimizing any visible
bulge under the skin at the site of device placement. The down-side of a
sub-pectoral implant is that it may involve more pain and potentially
more bleeding. Also, when it comes time to change the battery, the
operation can be a bit more involved. Other options for device placement
may also be considered, particularly when appearance is a concern; for
example, some women will prefer to have the device placed from the side
of the chest and under the breast.
The vein and the lead(s)
Subsequently,
a vein in the upper arm or upper chest is accessed; again, the
technique by which operators access this vein can vary. In some
circumstances, the operator will dissect the tissue down to the vessel.
More commonly, using known landmarks and a general knowledge of the
anatomy of the vein, the operator will introduce a needle on a syringe
into the vein. Sometimes, this is facilitated by a peripheral venogram,
a procedure where contrast dye that can be visualized under X-ray is
injected through an IV in the arm, enabling the operator to see the
filling of that vein as blood flows towards the heart. After the vein is
accessed with the needle, a floppy wire is placed through the needle
and well into the vein. The needle is taken out, leaving the wire in place, and a long plastic tube, termed a sheath,
is placed over the wire. The wire is then removed, leaving the sheath
in the vein. The sheath has a one-way valve in it, such that blood can
not flow from the vein outside, but other long wires or pacemaker leads can
be introduced into it from outside. After all of the air has been
removed from the sheath by drawing back blood, removing any air, and
flushing with fluid, a pacemaker lead is introduced through the sheath,
down the vein, and into the heart under visualization by an X-ray
camera.
The
floppy pacemaker lead has a hole in the middle throughout its length,
allowing for the placement of manually shaped and relatively stiff
stylettes (stiff wires) inside, providing support to the lead and a
means for the operator to steer it into position under X-ray guidance
(Figure 5). Depending on the patient’s underlying heart rhythm problem,
either 1 or 2 leads may be placed in different chambers of the heart:
for example, some patients will only have a single lead placed in either
the right atrium or the right ventricle and others may have one lead in
the right atrium and one lead in the right ventricle. In special
circumstances (described below in Section VII, a third lead may also be
placed). Note that the right heart chambers are used, primarily because
the veins provide relatively straightforward access to this side of the
heart. In addition, as reviewed in Section I, the venous and right sided
heart chambers do not supply blood directly to the brain; in the worst
case scenario that something should attach itself to a pacemaker lead
(such as a blood clot or collection of tissue from an infection) and
become dislodged, it is generally preferable that this occur on the
right side of the heart where debris can be caught by the lung rather
than on the left side of the heart where blood flow to the brain could
be blocked, potentially resulting in a stroke.
Figure 5.
Right atrial and right ventricular leads as visualized under X-ray
during a pacemaker implantprocedure. The atrial lead is the curved one
making a U shape in the upper left part of the figure.
Once in the position of interest, the pacemaker leads can be attached to the heart by 1 of 2 potential means: active fixation
leads have a screw at the tip that is deployed by turning a special
wrench at a particular spot on the other end of the lead remaining
outside the body; passive fixation leads have special tines on
them, allowing them to hook on the many trabeculations (nooks and
crannies) present on the inside of the heart (Figure 6). Regardless, it
is well understood that the real attachment of the leads requires the
patients own healing powers. Within approximately one month (although
the exact time can vary considerably from one person to the next), the
body will scar-down the tip of the leads, fixing them to the inside of
the heart.
Figure 6. Panel
A demonstrates 2 active fixation pacemaker leads, one with the screw
deployed (left). Panel B demonstrates 3 pacemaker leads with tines on
the end, so-called passive fixation leads that are designed to catch
onto muscular trabeculations in the heart. The figure in Panel A was
obtained with permission from Boston Scientific, Inc. (Natick, MA) and
the figure in Panel B was obtained with permission by Medtronic, Inc.
(Minneapolis, MN). Of note, all major pacemaker manufacturers supply both active and passive fixation pacemaker leads.
Once
the lead or leads are in position, they are tested electronically to
make sure they are working well. They are then secured by suture to the
floor of the pocket that has been made. The ends are then plugged into
the pacemaker generator.
The generator and closing up
The
pacemaker generator includes the battery and the computer, with all of
the pacemaker programming. It is typically the size of a thick 50 cent
piece or silver dollar (Figure 7). Once the lead or leads have been
plugged into the generator, they are secured there with a special screw.
Any residual bleeding is then typically addressed with cautery (using
an instrument that delivers small burns to scar the area and stop the
bleeding) or sutures, and the pocket is then typically washed with an
antibiotic and saline solution. The ends of the leads remaining outside
the body are then wrapped carefully behind the generator and the
generator is placed inside the pocket.
Figure 7.
An example of a pacemaker generator, with its dimension in millimeters
provided. This figure was obtained with permission from St. Jude
Medical, Inc. (St. Paul, MN).
The
pocket is then sewn together, typically in several layers to provide
strength and a good cosmetic result. For the final layer, some operators
will choose to use staples rather than sutures. Many operators will
often cover the sutures with special pieces of tape to help with the
integrity of the wound as it first heals, and almost all will place a
bandage over the wound that is typically removed later that same day or
the following morning.
Before going home
A
chest X-ray will typically be obtained after the procedure to make sure
that the pacemaker and leads remain in appropriate positions and to
make sure that there is no evidence of any complications related to the
lungs or heart (Figure 8). Typically, the pacemaker will also be
“interrogated.” This involves placing a special wand on the skin that
overlies the device, enabling communication with a computer. More
recently, many devices can be interrogated using wireless technology and
a wand is not needed. In either case, via this special pacemaker
computer, the battery life, programming, and integrity of the pacemaker
lead(s) can all be assessed. Depending on the operator and the
institution, some patients may be discharged home the same day as the
procedure assuming all goes well. In other cases, patients will
routinely be asked to remain over night and be discharged the following
morning (often with a second X-ray and the pacemaker interrogation that
morning to make sure everything remains normal).
Figure 8.
A normal chest X-ray after pacemaker placement, demonstrating the
pacemaker generator in the left upper chest attached to 2 leads (the
lower one in the right ventricle and the higher one in the right
atrium).
Partly
to protect the fresh wound and partly because the lead or leads are not
initially completely fastened to the heart (as above), the new
pacemaker patient will usually be instructed to restrict movement of the
arm on the same side as the pacemaker for the first week to first
month. For example, if the pacemaker is on the left side, the patient
may be instructed to avoid raising the left arm to shoulder level for
one week and above shoulder level for one month. Vigorous activity
should also generally avoided, particular heavy lifting using the arm on
the same side as the device, for at least a week. The patient will also
receive instructions regarding wound care, how long the incision needs
to remain dry, and any care of remaining tape and/or bandages.
Typically, an appointment to return to the outpatient clinic for a wound
check will be made in the first week and often the pacemaker will be
interrogated again within the first month.
Risks of the procedure
The
risks of the procedure are generally quite low. Nevertheless, as with
any procedure, physicians will typically recommend a pacemaker only when
the benefits outweigh the risks; that is, the physician believes the
risk of NOT having a pacemaker are greater than the procedure itself,
or, in cases where it is felt that quality of life may improve, that the
benefits outweigh the risks.
Although
the risks are very low, there are several possible complications: For
one, if the lung is injured when the needle is introduced into the vein,
air may enter into the cavity around the lung (the pleural cavity), resulting in lung collapse or a pneumothorax.
Usually, even if this happens, only a small amount of lung is affected
(which the patient sometimes does not even notice), and the lung
re-inflates itself without any intervention. As discussed above, this is
one reason to obtain a chest X-ray after the procedure; if a small
pneumothorax is seen, the patient may stay in the hospital for an extra
day or two for observation. More rarely, if the lung collapse is more
severe or very symptomatic, a chest tube may be placed via the side of
the rib cage in order to evacuate the air. Such a chest tube may stay in
for a few days, requiring a more prolonged hospital stay.
Bleeding
at the site is also a risk. Usually, because the pocket provides some
pressure and constriction to any blood that might build up, the risk
here is generally not related to blood loss per se. However, such a
build up of blood and clot (called a pocket hematoma) can be very
uncomfortable and also can increase the risk for infection. Sometimes,
this type of bleeding occurs in the setting of blood thinning medicines
that a patient needs to take for other reasons (such as a mechanical
heart valve or atrial fibrillation), and the decision to withhold versus
continue those blood thinning agents is made by the treating physician
in the hopes of maximizing benefit and minimizing harm.
Another
risk is infection, which is why the procedure is done under sterile
conditions and antibiotics are typically administered during – and often
after – the operation. Infection may occur through the skin site, and
may not show itself for a long period of time. After a pacemaker has
been in place, an infection from a different source that is traveling in
the blood can also attach itself to the pacemaker leads. Note that
these infections are typically severe bacterial or fungal infections (in
other words, not a common virus like the typical cold or flu). While
antibiotics are part of the treatment of a pacemaker infection, removal
of the pacemaker and leads is typically also necessary. As noted above,
due to the fixation of the leads to the heart by the bodies healing
process that occurs over time, pacemaker extraction can sometimes be
difficult and poses additional risks.
As
a final example, a rare but significant complication that can occur
during the procedure involves a perforation of the heart. If one of the
leads pokes a hole in the heart, bleeding can occur outside the heart,
collect in the sack that lines the heart (the pericardium), and
constrict the heart. This can be life threatening and the treatment
involves an emergency evacuation of the blood with a needle placed under
the rib cage. The hole will seal itself, and a drain placed into that pericardial space may have to remain in place for a few days; this requires that the patient stay in the hospital until the tube is removed.
It
is important to understand, however, that the great majority of
pacemaker procedures are done without any complications. Moreover, for
all of the complications listed above, there are solutions that can be
used to address the problem. The treating physician can also counsel
patients regarding the individual risks and benefits for a given patient as every situation is different.
IV. What does a pacemaker do?
As
mentioned above, a pacemaker prevents the heart from going too slow. In
some cases, it prevents the heart from stopping. The pacemaker lead is
capable of pacing when a small amount of electrical current is delivered from the generator: just enough energy is delivered to electrically capture
enough heart cells at the tip of the lead so as to result in the
generation of an electrical signal that can propagate throughout the
electrically connected heart chambers. Of note, this amount of energy is
so small that it typically is not felt by the patient (although the
patient might feel the heart beating, they will not experience any
sensation from the pacemaker lead directly). Rarely, if the pacemaker
lead is close to the nerve that supplies the diaphragm, the patient may
experience diaphragmatic stimulation, resulting in an
uncomfortable hiccup-like movement (another potential complication of
placement of the device). The leads also can record the electrical
activity of the heart that might occur due to the patient’s own heart
beat, and that recorded electrical activity can be communicated back to
the generator and thereby be detected or sensed. Finally, the pacemaker can react (and adjust) to the paced or sensed beats.
In
brief, through the pacemaker leads the device essentially can do three
things: pace, sense intrinsic heart beats, and react to the paced or
sensed beats. There are a few other functions that might be employed,
but these three activities are the primary building blocks. In fact, the
way physicians discuss the programming or mode of the pacemaker
involves an abbreviation that can immediately communicate the function
of these capabilities. This abbreviation scheme breaks down the
description of the programming into three letters: the first letter
describes where the pacemaker can pace, the second letter describes
where the pacemaker can sense an intrinsic beat, and the third letter
describes what the reaction to a sensed or paced beat will be (if any).
For
example, if a patient has a single lead in the ventricle that paces,
senses, and is inhibited from pacing if an intrinsic beat is sensed
would be described as having a pacemaker that is programmed to the VVI
mode: the first letter will be a “V” for ventricle (the pacemaker can
pace in the ventricle); the second letter will be a “V” (the pacemaker
can sense beats in the ventricle); and the third letter will be an “I”
for “inhibit” (when an intrinsic beat is sensed, it inhibits pacing). A
pacemaker that is programmed similarly with a lead only in the atrium
could be programmed to the AAI mode (“A” for atrium).
It
gets a bit more complicated when two leads are present. In such cases,
the most common programming is DDD (“D” here is for “dual”): the
pacemaker can pace in the atrium and the ventricle and the pacemaker can
pace and sense in the ventricle. The third “D” means that the pacemaker
is inhibited in a given chamber if it senses intrinsic activity in that
chamber (e.g., an intrinsic electrical signal in the atrium will
inhibit atrial pacing) and it means that the ventricular lead will track the atrial activity.
The
tracking process in dual chamber pacemakers also requires a bit more
explanation. After atrial activation (whether it’s a paced atrial beat
or sensed atrial activity), if no intrinsic ventricular activity is
sensed after a specified amount of time, the pacemaker will pace in the
ventricle. So if a patient has a dual chamber pacemaker programmed in
the DDD mode and the patient’s own electrical activity happens to be
working just fine for the time being, both intrinsic atrial and
ventricular activity will be sensed, the pacemaker will be inhibited,
and no pacing will occur. In fact, if the intrinsic conduction system is
working fine, pacing should indeed be avoided for two reasons: first,
it avoids unnecessary depletion of the battery and, second, it is
typically more healthy to allow the more organized electrical activity
of the heart’s native conduction system to activate the heart than to
generate an impulse from the pacemaker (see Section VII).
If
that same patient with a dual chamber pacemaker programmed to the DD
mode develops a very slow sinus rate such that the intrinsic heart rate
sensed in the atria and the ventricles drops below a pre-programmed lower rate limit (often
programmed to 60 beats per minute), the pacemaker will begin to pace.
Typically, the first paced beat will be in the atrium. If the AV node is
working well, that paced beat will travel throughout the atrium (much
like a heart beat of sinus node origin would), down the AV node, along
the specialized conduction system, and activate the ventricles. An AV delay
is programmed into the dual chamber device that is programmed to the
DDD mode: this is a period of time that begins when the atrium is paced
and will activate a ventricular paced beat only if a sensed ventricular
beat does not occur during that period of time. For example, the AV
delay may be set to 180 milliseconds (ms). If, after a paced atrial
beat, an intrinsic ventricular beat is sensed before the 180 ms have
elapsed, the device will inhibit ventricular pacing. However, if that
180 ms transpires without a sensed ventricular beat, the pacemaker will
track that atrial paced beat and follow it with a ventricular paced
beat.
Another
scenario occurs when the sinus node is working fine, but the AV node
conduction is slowed or blocked. In this circumstance (again, with a DDD
device), the intrinsic atrial beats will be sensed, inhibiting atrial
pacing. In addition to programming an AV delay (relevant to when the atrium is paced), a similar delay (sometimes called a PV delay)
is in place for intrinsic atrial beats. After sensing of the atrial
event, if a sensed ventricular signal occurs prior to the duration of
the PV delay, ventricular pacing will be inhibited; if, after an atrial
signal is sensed, the PV delay duration elapses without a sensed
ventricular beat, the pacemaker will track that atrial beat and deliver a
paced ventricular beat.
One other programming option that is useful to understand is called rate response.
Some will place an “R” as a fourth letter (e.g., DDDR) in order to
designate that this has been turned on. Rate response means that the
lower rate limit (the rate below which the pacemaker will disallow by
pacing) is adjusted depending on the patient’s activity. For example, if
the primary problem is chronotropic incompetence (discussed
above as the inability to mount an appropriately fast heart rate with
activity), simply disallowing the heart from going less than 60 beats
per minute probably will not be a lot of help when a patient is running
up a hill (in other words, the patient may remain very fatigued if the
heart rate does not increase above 60 beats per minute). Most pacemakers
are equipped with some sort of sensor of patient activity (either from
the heaviness of their breathing or the amount of movement or,
potentially even the content of components inside the blood stream) that
can reflect the degree of activity. With rate response, the pacemaker
can then increase the lower rate limit as needed in response to that
activity.
Of
note, there are many other pacemaker capabilities and programming
options that are beyond the scope of this review. Primarily, these are
complex topics pertinent primarily to the device manufacturers,
cardiologists, and cardiac electrophysiologists.
To
reiterate the point made at the beginning of this section (perhaps the
most important point), note that even in light of the relatively complex
discussion above, the pacemaker is only preventing the heart from going
too slow or from stopping. In other words, it only speeds up the heart
rate, it does not slow heart rates that are too fast. Addressing fast
heart rates is an entirely different (albeit sometimes related) topic
and typically requires medicines or, in some cases, other invasive
procedures such as catheter ablation or an implantable cardioverter-defibrillator.
V. What kind of care is needed after a pacemaker is placed?
The immediate care of a new pacemaker is described above in Section III under Before going home.
Otherwise, the only maintenance that is required involves coming in to
the local cardiologist’s or cardiac electrophysiologist’s office for
regular pacemaker checks or pacemaker interrogations. Typically,
after the initial few months after implant, these check-ups will be
scheduled approximately every six months. However, the exact scheduling
may vary considerably from one heart specialist to another.
During
these interrogations, a wand is typically placed on the skin overlying
the device. This wand is then connected to a computer specific to that
pacemaker manufacturer. Some of the more recently developed devices do
not require a wand as the devices can be interrogated using wireless
technology (as long as they are in somewhat close proximity). Most
cardiac hospital wards and certainly most hospital pacemaker clinics
have computers that will be able to recognize pacemakers made by each of
the different pacemaker companies. Alternatively, as these computers
(with their wands) are easily portable, someone from a given device
company can also be called to bring the necessary computer if one is not
available.
By
communicating with the pacemaker via this computer, the pacemaker
nurse, technician, device representative, or physician can do many
things. First, the battery can be checked. When only a few months are
remaining on the battery life, it is time to schedule a generator change
(described below). In addition, the integrity of the pacemaker leads
and the pacemaker generator can be assessed by running a variety of
tests involving assessments of sensing and pacing. Also, the pacemaker
mode and other special features can be reprogrammed as needed to
optimize the performance of the device for a given patient. Finally,
data about the patient, such as how fast their heart rate has been, how
often they require pacing, and sometimes the presence of other fast
abnormal heart rhythms can be detected.
Some
devices have special equipment to allow for pacemaker interrogations
from home, and some of the most recent features can perform such a
check-up without wires or without having to call the physician’s office.
Again, the degree to which these remote interrogations are used varies
by a given practitioner, but it is generally well accepted that they may
be best suited for those patients that live at great distances and/or
for whom frequent travel to the physician’s office is either not
feasible or is very inconvenient. Of note, someone (often a nurse or
pacemaker technician) still has to review and interpret any data that is
sent via these remote checks. With time, these automatic and remote
interrogation systems will continue to evolve, with the goal of catching
any problems with the pacemaker well before a scheduled check is due.
The generator change
As
mentioned above, pacemaker interrogations are helpful in providing data
on the battery life, and, when only several months are remaining on the
life of the pacemaker battery (or generator), it is time to schedule a
generator change procedure. The time from initial implant to generator
change can vary substantially and depends on several factors: how often a
patient is paced, the complexity of the pacemaker programming, and the
amount of resistance to each pacing impulse down the pacing lead (which
itself may be related to multiple patient and device factors). In
general, generator changes are required every few years (typically
somewhere between five and 10 years, but sometimes more and sometimes
less).
This
procedure is very much like the initial implantation, but generally a
more minor operation. After placing an IV and administering medicines to
help the patient relax and prevent general discomfort, the skin over
the device is infiltrated with local anesthetic (such as lidocaine or
xylocaine). An incision is made over the old device and, using various
instrument – such as cautery, scissors, a scalpel, and others – the
original pocket that was made (as described above in Section III) is
entered and the pacemaker generator is removed. The lead or leads
attached from that generator are unscrewed and taken out. Typically, the
leads are then attached via a cable to the computer so that their
function can be directly assessed. If there are any problems with a
lead, sometimes a new lead has to be placed. However, in the majority of
cases, problems with a lead would have been detected by the regular
interrogation described above and, if needed, a lead revision would have been anticipated by the physician and discussed with the patient before the procedure. Assuming
no new lead is needed, the inside of the pocket is washed with
antibiotic solution. Usually, the pocket will have formed dense scar
tissue and some believe that the risk of infection can be reduced if
this scar tissue is removed. The lead(s) are then screwed
into a new generator, that generator is placed into the pocket, and the
pocket and skin are sewn together (again, as described above in Section
III). Of note, the new generator is usually not the exact same model as
the one that has been removed. Given the time span between initial
implant (or previous generator change) and the generator change
operation in question, devices will have typically advanced as the
technology is essentially always moving forward. One of the more
recently available generators (with all of the accompanying new
programming and other new features) is compatible with the leads in
place and is typically used as the replacement. In addition, if the
implanting physician so chooses, the new generator can be chosen from a
different company (typically the different device company leads are
compatible with different device company’s generators as well).
Because
no new leads are placed during the great majority of generator
replacements, recovery from the procedure is generally quite quick.
Usually, patients will be sent home on the same day after the procedure
and a chest X-ray is typically not necessary. Because the leads are
generally well fastened given the scarring around them that occurs with
time, restrictions on arm movement are usually not as strict as those
given after the initial pacemaker implant.
The
risks of the procedure depend largely on whether a new lead has to be
placed. If a new lead is required, the risks are essentially the same as
those described above for an initial pacemaker implant (Section III).
One risk that is likely higher for the generator change procedure than
it is for the initial implant (regardless of whether new leads are
placed or not) is the risk of infection. Probably because the pocket has
a relatively poor blood supply given the natural scarring that occurs
with healing and time, the immune cells that can fight infection may not
be as readily available to the new generator as they were when the
pocket was first made. In the hopes of preventing
infection, most physicians will prescribe prophylactic (preventive)
antibiotics for several days after a generator change procedure (in
addition to the IV antibiotics routinely given during the operation).
VI. What kind of problems might be encountered due to a pacemaker?
With
time, damage can occur to the pacemaker (either the generator or
leads), resulting in malfunction or a complete loss of function. This
can occur with trauma to the chest or potentially from friction
occurring between the pacemaker leads and bones in the body. A pacemaker
may fail to capture (meaning the impulse generated from the
pacemaker is not able to electrically activate the heart) or fail to
sense (Figure 9). Often, these problems can be detected before they
result in any harm to the patient during the routine pacemaker checks,
and problems can therefore be averted by replacing the generator or
leads before failure occurs.
Figure 9. Panel A
illustrates atrial pacing: each pacing artifact (the sharp line denoted
by the black arrows) represents the electrical output from an atrial
lead and is followed immediately by a P wave, demonstrating that the
atrial lead is successfully capturing the atrium. In panel B, ventricular pacing is shown: the left side of this panel demonstrates the patient’s own rhythm, the grey arrow points to a fusion
beat (the ventricle is electrically activated by both the normal
conduction system and from the first ventricularly paced beat), and the
following beats result from ventricular pacing (note the asterix, which
denotes the small pacing artifact that can be seen preceding each of
those beats). Importantly, the ventricularly paced beats are wider than
the intrinsic beats: the swift and coordinated intrinsic conduction
electrically activates the ventricles in a rapid and organized fashion,
resulting in very abrupt electrical depolarization that is recorded as a
narrow QRS; in contrast, pacing from the right ventricle activates the
ventricles in a more slow, less organized, muscle cell to muscle cell
activation, resulting in a wide QRS (see text for discussion). Panel C
demonstrates pacing artifacts (black arrows) that are dissociated from
the patient’s rhythm, hence showing both failure to capture (as the
pacing stimuli do not affect the electrical activation of either
chamber) and failure to sense (as the pacing stimuli are not inhibited
as would otherwise be expected by the intrinsic beats).
Device Recalls
A
full discussion of device recalls and all of their implications is
beyond the scope of this review. However, while they are generally
uncommon, they are important enough to mention here. It must be
understood that, as can happen with any machine, pacemakers can develop
unanticipated problems. In fact, all device companies have had recalls
of their pacemakers at one time or another. Sometimes, the problem is in
the generator, sometimes the problem is in the lead. The severity of
the recall also varies: some problems can be fixed with a simple
software upgrade (via the computer in a non-invasive fashion) and others
require a surgical procedure for replacement. Fortunately, on the
whole, these recalls are rare, but patients with pacemakers should
realize that a device recall (as with any complex device) is a
possibility.
Electromagnetic Interference or “Can I still use my microwave?”
Electromagnetic interference (EMI) is essentially any high powered electric or magnetic signal that is sensed by a pacemaker.3
Fortunately, given the improved shielding of commonly used devices as
well as an improvement in the way modern pacemakers are made EMI with
pacemakers is quite rare. However, it is important to understand what
EMI can do and to be familiar with common sources of EMI that might be
encountered. An exhaustive list will not be provided here, but the
pacemaker technician or nurse that performs the pacemaker interrogation
at regular intervals and/or the representative from the company that
makes a particular device are good resources for this information.
Briefly,
while modern microwave machines are not a problem around modern
pacemakers, some modern-day sources of EMI can include cell phones (in
particular situations as below), arc-welding devices, and security
devices such as those encountered at the airport. Many other potential
sources of EMI are found in the hospital and involve other procedures,
such as lithotripsy, electroconvulsive therapy, electrical
cardioversions, and magnetic resonance imaging. Fortunately, in all of
these cases, a physician is involved and will help make decisions as
well as communicate with the heart specialist responsible for a
patient’s general pacemaker care. Because they are relatively common and
are true sources of particularly strong EMI, MRI machines are discussed
briefly below.
The
concern is that, if an electromagnetic signal is detected by a
pacemaker, that pacemaker generally has no way of knowing that the
signal originates from somewhere outside the patient’s heart. Therefore,
it may interpret this outside signal as intrinsic heart activity. If
this signal is interpreted as arising from the atrium, the pacemaker may
be programmed to track that signal in the ventricle, potentially
resulting in an inappropriately fast rate. More commonly and more
concerning, the signal may be interpreted as arising in either the
atrium or the ventricle and inhibit the pacemaker, such that pacing
signals are not delivered. In other words, the pacemaker is programmed
to do certain things when it senses electric signals (for example, as
described above in Section IV, pacemakers are generally programmed to
inhibit ventricular pacing, or not pace in the ventricle, if a patient’s
own conduction system activates the ventricle), but it is not smart
enough to differentiate intrinsic electrical signals originating from
the heart’s native conduction versus EMI signals that may arise from
external machinery. If the signal is inhibited in a person who only has
intermittently slow rhythms and the rhythm happens to be normal at the
time, it is likely that there will be no problems. However, if the
patient is dependent on the pacemaker and has significant heart block or
a dangerous absence of his own rhythm, such inhibition from the
external signal can result in a loss of consciousness or potentially
even death. It is important to understand that the effect generally
lasts only as long as the pacemaker is detecting the external activity;
the pacemaker should resume normal activity as soon as it stops
detecting external signals.
So
consider the metal detector/security device people walk through at the
airport – or the wand that airport personnel sometimes use. Both of
these devices emit EMI. If one walks through and does not linger under
the detector, there is not enough time for the EMI to inhibit pacing for
a sufficiently long duration to cause problems. Similarly, if the wand
is swept over the area of the device, a fraction of a second of EMI will
not be long enough to inhibit pacing such that poor perfusion to the
brain or other organs will be compromised. However, if a
pacemaker-dependent patient stood under the metal detector for several
minutes, the constant EMI might inhibit the pacemaker during that entire
time, potentially resulting in no heart beats (assuming the patient is
completely dependent on the pacemaker, with no intrinsic activity at
all).
Cell
phones are generally not felt to emit sufficient energy to inhibit
pacemakers when carried at a reasonable distance from the pacemaker.
However, it is generally recommended that they not be carried in a
pocket directly over the device. The strongest signal is thought to
occur just when the cell phone is called, and, if a cell is called while
sitting in a pocket directly over the device, it could result in
significant EMI. Arc-welding is mentioned above simply because it
reliably results in significant EMI and therefore pacemaker patients
should generally avoid being near that activity.
As
mentioned above, EMI generally does not permanently damage pacemakers,
but tends to wreck havoc only while it is present. However, very
powerful electromagnetic fields can theoretically permanently affect a
pacemaker. One example of this is magnetic resonance imaging (or an
MRI), which is often used to image a certain part of the body for
medical reasons. Fortunately, recent data suggests that on the whole
modern pacemakers generally appear to be protected against significant
damage due to MRI machines, but close monitoring is required and the
decision for a pacemaker patient to undergo MRI generally requires a
discussion amongst the physicians caring for the patient (typically
including the radiologist and cardiac electrophysiologist). It is
generally recommended that pacemakers be interrogated before and after a
patient has undergone imaging with an MRI machine.
VII. Biventricular pacemakers
As
discussed above, the purpose and function of the majority of pacemakers
is to prevent the heart from beating inappropriately slow. Biventricular pacemakers, also called cardiac resynchronization therapy (or CRT),
are a major exception. In most cases, the function of biventricular
pacemakers has little to nothing to do with the heart rhythm, but
instead helps weak and uncoordinated hearts to work more efficiently.4
These devices are generally reserved for patients who have symptomatic
heart failure due to a weak heart. Symptoms of heart failure typically
include difficulty breathing, decreased exercise tolerance, fatigue and,
often, swelling in the legs. Generally these conditions are due to a
build up of fluid in the lungs and other tissues of the body because of
difficulty filling the heart, as well as an inability to provide a
sufficient forward flow of blood from the heart because of weakened
pumping action. While the physical exam can be helpful in determining if
a patient has a heart that does not pump as strong as it should,
typically an ultrasound of the heart (an echocardiogram) and/or other imaging (such as a nuclear test [such as a SPECT, MUGA, or PET
scan] or MRI) is required to document this. Importantly, in order to
qualify for one of these devices, these patients generally also need to
have a QRS (the deflection on the electrocardiogram that represents
electrical activation of the ventricle as described in Section II) that
is particularly wide. The reasons for this will be explained below.
A
substantial number of patients with heart failure due to a weakened
heart will have what is called a bundle branch block. In this situation,
one of the bundle branches (as described in Section II and illustrated
below in Figure 10) is not able to conduct the electrical signal
normally. In that situation, the electrical signal progresses down the
AV node, down the bundle of His, and down the now single bundle branch
that remains working (remember that if both bundle branches are
completely dysfunctional, complete AV block will occur as described in
Section II). Once that impulse arrives at the end of that
still-functioning bundle branch, it will then activate the side of the
heart that normally would have been activated by the now dysfunctional
branch via muscle cell to muscle cell conduction. Importantly, because
that activation does not occur through the normal conduction system, but
instead from heart muscle cell to heart muscle cell, the electrical
conduction is not as rapid nor as organized. For example, if the left
bundle branch is blocked (which can often happen due to the same heart
disease that has resulted in the weakening of the heart in heart failure
patients), the electrical impulse arising from the AV node will travel
down the bundle of His and down the right bundle branch. The right
ventricle will then be activated normally. Subsequently, the left
ventricle will be activated from muscle cell to muscle cell from the
right side (where the signal is now originating) to the left side. As
the contraction of the heart follows the same pattern as the electrical
activation, this then means that the left ventricle will undergo
contraction of the septum first (the area closest to the right
ventricle) and the lateral (most left-ward) wall last. Hence, the left
ventricle will contract in a dyssynchronous fashion. This is in stark
contrast to the synchronous contraction that occurs when the left bundle
branch is intact: in this normal situation, electrical conduction
travels down the left bundle branch which delivers the electrical signal
via its many branches throughout the left ventricle, activating it in
an efficient and synchronized way, resulting in a squeezing action with
all walls of the left ventricle working in concert. In the setting of a
heart that is already weak, a left ventricle (the main chamber pumping
blood to the brain and tissues of the body) pumping in such a
dyssynchronous manner can worsen all of the symptoms of heart failure.
It is important to understand that many people without heart failure may
have a bundle branch block without any ill effects. In addition, some
heart failure patients may have true dyssynchrony with a narrow (or
normal appearing) QRS. Currently however, the QRS is used as the primary
indicator of underlying dyssynchrony in heart failure patients.
Figure 10. The conduction system of the heart. This figure was obtained with permission from Mr. David Criley at www.blaufuss.org.
When
a right ventricular pacing lead delivers an electrical stimulus,
electrical conduction occurs from the right ventricle, primarily through
heart muscle tissue, throughout the right ventricle and then on
similarly to the left ventricle. Therefore, pacing from the right
ventricle (as is typically done in the majority of ventricular
pacemakers indicated for slow heart rhythms) actually can induce
dyssynchrony (for the same reasons a left bundle branch block would,
with conduction occurring via muscle cell to muscle cell rather than via
the specialized conduction system). In fact, there is now evidence that
this may worsen heart failure in patients, particularly in those who
already have some degree of heart function decline.5
Therefore, most current pacemaker algorithms are designed to help to
avoid right ventricular pacing by allowing natural ventricular
conduction to occur as frequently as possible.
What can be done for the heart failure patients with dyssynchrony? A biventricular device can help to resynchronize
the heart. One important component of a biventricular pacemaker
involves the placement of a right ventricular lead and potentially right
atrial lead just as described for regular pacemakers above. In
addition, a third lead is placed. Accessing the vein in the arm as
described above (Section III), a long sheath or hollow catheter is maneuvered into a special vein called the coronary sinus.
The coronary sinus drains the blood from the heart into the lower right
atrium. This vein wraps around the outside of the left side of the
heart and provides branches to the left ventricle. By accessing the
coronary sinus under X-ray guidance with a long sheath, a long floppy
pacemaker lead can be maneuvered into one of those branches (often over a
long, thin wire) and left in place directly overlying the outside of
the left ventricle (and ideally the lateral or most left-ward side of
the left ventricle).
Often
times, the coronary sinus is injected with dye that can be seen under
X-ray in order to view the branches just prior to placing the lead. That
long sheath can then be peeled away and the coronary sinus lead is then
attached to a special biventricular device that has ports for three
leads (the coronary sinus, right ventricular, and, in most cases, right
atrial leads, Figure 11). By simultaneously or
near-simultaneously pacing the right ventricular and coronary sinus (or
left ventricular) leads together, the left ventricle is then activated
to squeeze in a synchronous manner, helping to restore efficiency and
strength of its pumping action. In fact, this therapy has been shown to
improve quality of life, the strength of the heart, and reduce
hospitalizations4 and even death6 in patients with heart failure due to a weak heart in the setting of a wide QRS complex.
Figure 11. Three
leads can be seen in this example of a cardiac resynchronization
device: a right atrial lead (solid black arrow), a right ventricular
lead (dashed black arrow), and a coronary sinus lead (red arrow). The
coronary sinus lead wraps around the outside of the left ventricle,
enabling pacing of the left ventricle. Note that the right ventricular
lead in this case has 2 thickened aspects that represent conduction
coils and that the generator is larger than typical pacemaker
generators, demonstrating that this device is both a pacemaker and a
cardioverter-defibrillator, capable of delivering electrical shocks for
dangerously fast abnormal ventricular rhythms (see separate knol on
Implantable Cardioverter-Defibrillators (ICDs).
Other resources:
An excellent review of indications for pacemaker placement provided by the American Heart Association, American College of Cardiology, and North American Society of Pacing and Electrophysiology (now the Heart Rhythm Society) can be found at http://www.americanheart.org/downloadable/heart/1032981283481CleanPacemakerFinalFT.pdf . The Heart Rhythm Society has a website dedicated to the treatment of abnormal heart rhythms (at www.hrsonline.org). Patient pages from this site can be found directly at http://www.hrspatients.org/patients/signs_symptoms/default.asp
To learn more about the treatment of arrhythmias at the University of California, San Francisco (UCSF), please visit www.ucsfhealth.org/arrhythmia
References
1. Gregoratos
G, Abrams J, Epstein AE, Freedman RA, Hayes DL, Hlatky MA, et al.
ACC/AHA/NASPE 2002 guideline update for implantation of cardiac
pacemakers and antiarrhythmia devices: summary article: a report of the
American College of Cardiology/American Heart Association Task Force on
Practice Guidelines (ACC/AHA/NASPE Committee to Update the 1998
Pacemaker Guidelines). Circulation 2002;106(16):2145-61.
2. Mangrum JM, DiMarco JP. The evaluation and management of bradycardia. N Engl J Med 2000;342(10):703-9.
3. Yerra L, Reddy PC. Effects of electromagnetic interference on implanted cardiac devices and their management. Cardiol Rev 2007;15(6):304-9.
4. McAlister FA, Ezekowitz J, Hooton N, Vandermeer B, Spooner C, Dryden DM, et al. Cardiac
resynchronization therapy for patients with left ventricular systolic
dysfunction: a systematic review. JAMA 2007;297(22):2502-14.
5. Wilkoff
BL, Cook JR, Epstein AE, Greene HL, Hallstrom AP, Hsia H, et al.
Dual-chamber pacing or ventricular backup pacing in patients with an
implantable defibrillator: the Dual Chamber and VVI Implantable
Defibrillator (DAVID) Trial. JAMA 2002;288(24):3115-23.
6. Cleland
JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L, et al.
The effect of cardiac resynchronization on morbidity and mortality in
heart failure. N Engl J Med 2005;352(15):1539-49.