 NIH: Can
over-the-counter hearing aids for older adults with mild-to-moderate
hearing loss work as well as hearing aids purchased through an
audiologist? In a new study, participants who selected pre-programmed
hearing aids using an over-the-counter delivery model reported a similar
level of benefit as participants who purchased the same hearing aids
through an audiologist following best practices. The audiology services
included fitting the hearing aids and counseling the consumers on how to
use them. The National Institute on Deafness and Other Communication
Disorders (NIDCD), part of the National Institutes of Health, supported
the study, which is published in the March 2 American Journal of Audiology.
NIH: Can
over-the-counter hearing aids for older adults with mild-to-moderate
hearing loss work as well as hearing aids purchased through an
audiologist? In a new study, participants who selected pre-programmed
hearing aids using an over-the-counter delivery model reported a similar
level of benefit as participants who purchased the same hearing aids
through an audiologist following best practices. The audiology services
included fitting the hearing aids and counseling the consumers on how to
use them. The National Institute on Deafness and Other Communication
Disorders (NIDCD), part of the National Institutes of Health, supported
the study, which is published in the March 2 American Journal of Audiology. The study is the first randomized, double-blind, controlled clinical trial to compare the efficacy of two service-delivery models of hearing aids. Led by Distinguished Professor Larry Humes, Ph.D., of the Department of Speech and Hearing Sciences at Indiana University Bloomington, the research team followed for six weeks 154 participants, aged 55 to 79, with mild-to-moderate hearing loss. All participants received a thorough hearing evaluation before being randomly assigned to one of the service-delivery model groups to purchase the hearing aids.
The researchers compared standard measures of benefit, user satisfaction, and usage of the hearing aids after six weeks. To assess the efficacy of the hearing aids based on service-delivery model as well as on purchase price, the researchers compared outcomes of participants in the two service-delivery models with a third group of volunteers who purchased “sham” hearing aids from the audiologists. These devices were programmed to offer no amplification, thus serving as a control for the study.
The researchers found the hearing aids to be efficacious in both service-delivery model groups, compared to the control group. In addition, participants in the over-the-counter group had “only slightly poorer outcomes” than those in the audiology service delivery group.
“Efficacious over-the-counter service-delivery models (and devices) may increase accessibility and affordability of hearing aids for millions of older adults,” the authors write. “Further research is required to evaluate various devices and approaches, as well as to examine the generalization of the findings from this clinical trial.”
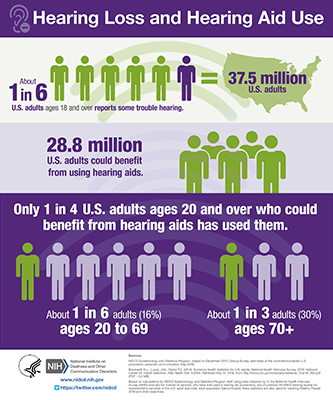
This study is one of 27 clinical and translational research projects throughout the United States that the NIDCD funds to develop and test novel strategies to overcome barriers to hearing health care. Improving hearing health care for adults is an urgent public health problem, as only one in four of the estimated 28.8 million adults in the U.S. who could benefit from hearing aids has used them.
In addition to supporting clinical and basic research studies, the NIDCD cosponsored a consensus study conducted by the National Academies of Sciences, Engineering, and Medicine (link is external) to identify priorities for improving access and affordability of hearing health care for adults. Released June 2, 2016, the report provides a thorough analysis of the state of hearing health care for adults in the U.S. and puts forth 12 recommendations to direct hearing health care reform. The NIDCD is one of several federal agencies working with the National Academies and stakeholders to implement the report’s recommendations.
The hearing aid clinical trial was supported by NIH grant DC011771. Details of the study are available at clinicaltrials.gov, NCT01788423.
Read the press release from the American Speech-Language-Hearing Association (link is external).