Unlike traditional light microscopy, EM uses electrons, not light, to create an image. To do so, EM accelerates electrons in a vacuum, shoots them out of an electron gun and focuses them with doughnut-shaped magnets onto a sample. When electrons bombard the sample, some pass though without being absorbed while others are scattered. The transmitted electrons land on a detector and produce an image, just as light strikes a detector (or film) in a camera to create a photograph.
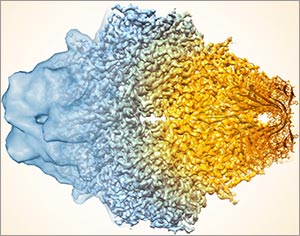
Transmission electron microscopes can magnify objects more than 10 million times, enabling scientists to see the outline and some details of cells, viruses and even some large molecules. A relatively new form of transmission electron microscopy called cryo-EM enables scientists to view specimens in their natural or near-natural state without the need for dyes or stains.
In cryo-EM—the prefix cry- means “cold” or “freezing”—scientists freeze a biological sample so rapidly that water molecules do not have time to form ice crystals, which could shove cellular materials out of their normal place. Cold samples are more stable and can be imaged many times over, allowing researchers to iteratively refine the image, remove artifacts and produce even sharper images than ever before.
Thanks to a new generation of detectors and improved image-processing software, cryo-EM enables extremely high magnification. The level of detail, or resolution, of cryo-EM imagery now rivals that obtained with X-ray crystallography, which provides the exact location in space of every atom in a molecule (though under conditions that are far from natural).
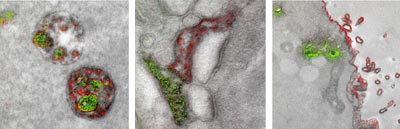
After
decades of black and white electron microscopy images, scientists now
have a way to incorporate color. Credit: Adams et al., Cell Chemical Biology 2016.
Historically, EM images have only been in black and white. In late 2016, a group of researchers that included the late Roger Tsien—who won a Nobel Prize for bringing color to light microscopy using green fluorescent protein—published the first ever color EM images. The technique uses rare earth metals called lanthanides to label different biomolecules. This multicolor EM is already providing scientists with previously unobtainable information about cell structure, protein movements within and between cells, and views of cell components at a level of detail not possible with light microscopy.