Author: Dr S Andrew Josephson University of California San Francisco 2012-02-20
Introduction
Introduction
A stroke is defined as the sudden onset
of a neurologic deficit attributable to a vascular cause. A stroke
results from lack of blood flow to an area of the brain. Without
adequate blood flow, neurons (nerve cells) in the brain will begin to
die. Symptoms of a stroke are variable depending on the area of the
brain involved but can include weakness or numbness on one side of the
body, new problems with vision, dizziness, or severe headache. A stroke
is classified as either (1) ischemic (also termed “cerebrovascular
accident” [CVA]), where an occluded blood vessel deprives an area of the
brain of blood flow
or (2) hemorrhagic (also termed intracerebral hemorrhage [ICH]), where
there is bleeding into the brain tissue itself; approximately 15-20% of
strokes are hemorrhagic in nature and these are the focus of this
review.
Nearly 75,000 patients suffer an ICH each year in the
United States. It is a tremendously deadly disease: an estimated 35 to
50% of patients die within one month of their ICH. Rapid recognition and
treatment of ICH and its complications is essential in an attempt to
reduce disability and death. Because ischemic stroke and ICH can have
similar symptoms, brain imaging with computed tomography (CT) or
magnetic resonance imaging (MRI) is necessary to identify those patients
with hemorrhage. The similarity in clinical presentation of ischemic
stroke and ICH means that all stroke patients are initially triaged and
treated similarly in the emergency setting until head imaging can make
this important distinction.
Etiology of ICH
Once
an ICH has been identified, much of the early diagnostic approach
focuses on determining the etiology of the hemorrhage. Trauma to the
head may lead to ICH that is small or massive in volume. By far the most
common cause of non-traumatic ICH is uncontrolled hypertension (about
60-70% of spontaneous ICH). Hypertensive-related ICH can occur in any
location in the brain but has a particular predilection for the putamen
in the basal ganglia, the thalamus, the cerebellum, and the pons in the
brainstem (Figure 1). Hypertensive-related ICH should be thought of as a
diagnosis of exclusion, once other etiologies have been ruled-out. In
order to exclude other causes of ICH, a variety of additional imaging
tests may be employed, including MRI with contrast, CT or MR
Angiography, and conventional catheter-based angiography.
Figure 1:
Non-contrast CT scans of the brain demonstrating typical locations of
hypertensive-related ICH: (A) thalamus, (B) putamen (in the basal
ganglia), (C) pons (in the brainstem), and (D) cerebellum
Underlying
congenital malformations of the blood vessels (“vascular
malformations”) are another important etiology of ICH, especially in
younger patients without a history of hypertension. Arteriovenous
malformations (AVMs) are abnormal tangles of blood vessels that can be
asymptomatic prior to rupture and are usually diagnosed via angiography
(Figure 2). Cavernous malformations are a distinct type of vascular
malformation that can lead to ICH and are typically identified via MRI
(Figure 3). Although ruptured aneurysms may lead to ICH, the more common
presentation is that of a subarachnoid hemorrhage (SAH) where blood
pools in the spaces surrounding the brain rather than in the brain
parenchyma itself [ref SAH knol].
Figure 2:
Cerebral catheter-based angiogram of a 38-year-old woman, who presented
with a lobar ICH, demonstrating an abnormal tangle of vessels that was
removed surgically and found to be an arteriovenous malformation (AVM).
Figure 3:
T2-weighted brain MRI demonstrating a cavernous malformation (arrow)
with surrounding edema. The patient had presented 4 months prior with an
ICH in the same location, but initial MRI failed to demonstrate this
lesion as it was obscured by blood at the time.
Other underlying masses in the brain, such as brain tumors or
infectious abscesses, may present with ICH (Figure 4). Initial MRI or CT
may not be able to identify these lesions, as blood may obscure their
visualization; often repeat scanning, months later, when blood has
spontaneously absorbed, will allow for identification of these masses.
Figure 4:
Non-contrast CT scan of the brain demonstrating a left-sided lobar ICH.
MRI and brain biopsy revealed the etiology to be from an underlying
metastatic tumor in the setting of newly-diagnosed renal cell carcinoma.
Abuse of sympathomimetic
drugs such as cocaine and amphetamines can lead to ICH, making
toxicology screening an important part of initial ICH evaluation. These
compounds lead to ICH both due to their effects on systemic blood
pressure, leading to severe transient hypertension, and due to their
weakening effects on the walls of cerebral blood vessels with repeated
use.
Other less common causes of ICH
include amyloid angiopathy, a disease of the elderly where blood vessel
walls are weakened due to deposition of an abnormal protein;
coagulopathy, an increased bleeding tendency from disorders such as
liver disease, malignancy, or medications used to thin the blood;
ischemic stroke with secondary hemorrhage; and inflammatory disorders
involving the blood vessels of the brain (“vasculitis”).
Emergency Evaluation of ICH
Patients
with suspected stroke presenting to the emergency department (ED) are
quickly imaged with CT or MRI after initial stabilization in order to
distinguish ischemic stroke from ICH1. Although MRI is likely
as sensitive for identifying ICH, it is much more time consuming and
requires more patient cooperation, therefore most hospitals use CT
scanning as the initial imaging modality for patients with suspected
ICH.
The evaluation of patients with ICH
focuses on identifying ICH risk factors in an attempt to discern the
etiology of the hemorrhage. The physical examination looks for signs
that may indicate head trauma such as lacerations or fractures. Blood
pressure elevation after ICH is common and may provide a clue that
hypertension or drug abuse is responsible. Laboratory investigations
should focus on excluding systemic coagulopathy with measures of
clotting parameters and platelet count. A urinary toxicology screen is
important in all patients with ICH in order to rule-out drug-related ICH
from cocaine or amphetamine abuse. A careful medication history
including over-the-counter and herbal substances is important as some of
these compounds have either sympathomimetic properties (such as
ephedrine) or interfere with normal coagulation, increasing the risk of
ICH.
The results of initial imaging may
provide clues as to the etiology of ICH. Hemorrhages in the deep nuclei
of the brain including the putamen and thalamus, along with the pons
and cerebellum, are more likely to be hypertensive in etiology while
lobar hemorrhages near the surface of the brain may have an alternative
etiology such as underlying vascular malformation or amyloid angiopathy.
Despite these tendencies, there remains substantial overlap in location
of ICH from various causes making it difficult to determine etiology
based on ICH location alone. Imaging studies early in ICH can also
identify hydrocephalus or other signs of increased intracranial pressure
(ICP) that may necessitate emergent therapy (see below).
A
number of authors have attempted to devise models that can predict
outcome in ICH in order to allow for more effective communication with
families. The ICH score is one widely-used, simple, validated method
that can be calculated using information readily available to emergency
medical personnel shortly after ICH presentation (Table 1)2.
The ICH score has been shown to correlate well with 30-day mortality;
those with higher scores are more likely to die in the first month
following ICH.
TABLE 1: The ICH score for predicting 30-day mortality
Component Points
Glasgow Coma Scale score (GCS)
3-4 2
5-12 1
13-15 0
ICH Volume (cc) on CT 3-4 2
5-12 1
13-15 0
≥ 30 1
< 30 0
Intraventricular Hemorrhage (IVH) < 30 0
Yes 1
No 0
Infratentorial Origin of ICH No 0
Yes 1
No 0
Age (years) No 0
> 80 1
< 80 0
< 80 0
Total ICH Score 0-6
GCS = GCS score on initial presentation (or postresuscitation);
IVH = presence of any intraventricular hemorrhage on initial CT
Medical Management of ICH
There
are currently no U.S. Food and Drug Administration (FDA)-approved
treatments for ICH. Patients with ICH should be managed initially in an
intensive care unit (ICU) allowing for close, frequent monitoring of the
neurologic and medical condition of the patient. Since hypertension is a
common cause of ICH, management of high blood pressure remains the
central treatment early after a hemorrhage. Most experts agree that
lowering the blood pressure of patients with ICH decreases the chances
of continued bleeding and expansion of hemorrhage. Intravenous,
short-acting blood pressure-lowering agents are usually initially used
in the ICU, followed by a transition to oral long-acting blood pressure
medications prior to discharge from the hospital. If blood pressure is
reduced too aggressively, there is at least the theoretical risk of
secondary ischemia due to decreased blood flow to areas of injured
brain. Current trials are ongoing to determine the most appropriate
blood pressure goals after ICH3; for now, published recommendations endorse a modest reduction of blood pressure in the acute setting1.
Patients
who are found to have coagulopathy should have rapid reversal of these
blood clotting abnormalities in order to prevent continued hemorrhage
expansion. Patients with low platelet counts should receive a platelet
transfusion. Those with coagulopathy secondary to treatment with
warfarin should be reversed using vitamin K or fresh frozen plasma in
order to replete clotting factors that have been inhibited by this
medication. It has been recognized recently that coagulation
abnormalities in the setting of warfarin can be reversed much more
rapidly, and with smaller volumes of fluid, using compounds containing
high levels of purified or recombinant clotting factors such
as prothrombin complex concentrate4. Future trials will be needed to weight the benefit of this more rapid strategy against the high cost of these medicines.
Studies of ICH patients without
coagulopathy who undergo serial imaging using CT scanning have
demonstrated that nearly one-third experience growth of their hematoma
in the first hours following an ICH. Hematoma growth is associated with
poor outcome5. This has led to trials of activated factor VII
(rFVIIa), another compound containing important clotting factors that
rapidly stops bleeding throughout the body, in non-coagulopathic
patients with ICH. An initial study of this approach showed great
promise, with decreased rates of hematoma growth and decreased mortality
among patients treated with rFVIIa6; unfortunately, the large follow-up trial designed to confirm these results demonstrated no benefit of the treatment11.
Nonetheless, this strategy of administering therapies in order to
prevent hematoma growth in patients with ICH, perhaps in carefully
selected patients, merits future investigation.
High
glucose levels (hyperglycemia) and high core body temperature in
patients with ICH have each been associated with worse outcomes and
should be treated aggressively. Control of hyperglycemia in the ICU
usually involves administration of insulin as an intravenous continuous
infusion or subcutaneous injection along with frequent measurement of
serum glucose levels. Fever control can be accomplished with medications
such as acetaminophen or through use of external or internal cooling
devices.
Seizures occur in 4-8% of
patients with ICH and are more common with lobar (superficial) ICH
location. Once seizures occur in a patient with ICH they should be
treated aggressively with anti-epileptic medications. Patients with
seizures and ICH should be discharged from the hospital with
anti-epileptic drugs for at least 3-6 months in order to prevent further
seizures. A more controversial issue is whether all ICH patients should
be treated with anti-epileptic medications in order to prevent the
initial occurrence of seizures. There is little evidence to support this
strategy, and enthusiasm for prevention of seizures should be tempered
by the side effects of these medications and the relatively low
frequency of seizures following most ICHs. Currently, the decision to
administer prophylactic anti-seizure medications varies widely by
institution and individual physician.
Patients
with ICH are also at risk for multiple medical complications during
their hospitalization. The immobility that accompanies ICH puts patients
at risk for deep venous thrombosis and pulmonary embolus; prevention
can be accomplished with either pneumatic compression devices on the
legs or subcutaneous heparinoid compounds (the latter may be begun 3-4
days after ICH with clear documentation that bleeding has stopped).
Adequate nutritional support, via a feeding tube if the patient cannot
swallow, has been shown to improve outcome in all types of stroke and
should be begun within the first 24-48 hours. Physical, occupational,
and speech therapy should be instituted early and aggressively during
the course of the hospitalization in order to begin the process of
rehabilitation and recovery.
Surgical Management of ICH
Surgical
removal of blood clot in the brain would seem to be an intuitive
approach to treatment of ICH, but this strategy may injure normal
surrounding brain tissue and exposes patients who are already quite ill
to a major neurosurgical procedure. A single multicenter randomized
trial, the International Surgical Trial in Intracerebral Hemorrhage
(STITCH), attempted to clarify the role of surgery in ICH7.
The results of this study did not show any benefit for surgical
evacuation of clot in ICH compared with medical management alone.
Subgroup analysis suggested that there may be a trend toward benefit
from surgery only in the fairly rare case of lobar ICH located within 1
cm of the surface of the brain. Therefore in most cases of ICH, there is
no role for surgery, and the hematoma will slowly absorb spontaneously
over time. More less invasive surgical approaches are currently being
examined in large trials.
An important
exception to the lack of proven benefit from surgery in ICH involves
hemorrhages in the cerebellum. Cerebellar hematomas cause particular
difficultly as swelling can lead to rapid deterioration and death due to
obstruction of the fourth ventricle and pressure on the brainstem,
which contains structures vital to maintaining alertness and the ability
to move and breathe. Patients with cerebellar ICH were not included in
the STITCH trial, and it is generally recommended that all patients with
cerebellar ICH greater than 3 cm in diameter who experience neurologic
deterioration should undergo removal of the clot as soon as possible.
Management of Elevated ICP and Hydrocephalus
When
blood leaks into the brain during an ICH, one of the most devastating
consequences is elevation of intracranial pressure (ICP), and prompt ICP
treatment likely leads to improved outcomes. Patients with increased
ICP may present with somnolence, headache, vomiting, or a progressively
worsening neurologic exam. Because increased ICP may occur anytime in
the hours to days following ICH, patients must be monitored carefully,
with frequent assessments of neurologic status in an ICU setting.
One
important etiology of increased ICP in ICH patients is hydrocephalus,
where the fluid-filled ventricles in the brain expand and put pressure
on the rest of the brain parenchyma due to an inability to properly
drain. Anytime that blood is deposited in the normal clear fluid of the
ventricles, hydrocephalus can result. Hydrocephalus can be recognized
with CT or MRI imaging of the head (Figure 5). Treatment of
hydrocephalus usually involves placing a drain (“ventriculostomy”)
through the skull in order to allow cerebrospinal fluid (CSF) to drain
externally in a controlled manner. Some patients will only require
drainage for a short period of time; however, some ICH patients remain
unable to drain CSF and require placement of a permanent internal shunt
that drains CSF into various body cavities such as the abdomen (termed a
ventriculoperitoneal shunt [VPS]).
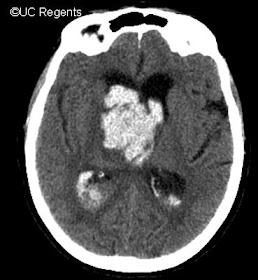
Figure 5:
Non-contrast CT scan of the brain demonstrating a right-thalamic ICH
with extension of blood into the ventricles and resulting hydrocephalus.
A ventriculostomy was placed shortly after this scan to allow for
adequate ventricular drainage.
The other
common cause of increased ICP aside from hydrocephalus involves swelling
(edema) that occurs in the brain tissue surrounding the hemorrhage.
Edema usually peaks during the first 3-5 days after an ICH, but lack of
effective edema management during these first few days may lead to
permanent disability, or even death. Treatment of increased ICP in
patients with ICH begins with simple but effective measures, including
raising the head of the bed and avoiding fluids that contain a high
concentration of water such as D5W and half-normal saline. Adequate
sedation and pain control can prevent increases in ICP that occur with
patient discomfort.
If ICP remains elevated despite these simple
measures, a more aggressive approach is indicated. Although no good
trials exist to support this approach, an ICP monitor is usually placed
in order to allow physicians to continually measure the ICP and its
response to therapy. Most ventriculostomy devices used to treat
hydrocephalus also permit accurate intermittent measurement of ICP in
addition to drainage.
Hyperventilation is an effective method of
rapidly reducing ICP in patients who are mechanically ventilated, but
its use is limited by its very transient effect. It also causes a
simultaneous lowering of cerebral blood flow, potentially leading to
cerebral ischemia. As a result, hyperventilation should be used only on a
very temporary basis in patients with elevated ICP8.
Osmotic
therapy, using mannitol or hypertonic saline, remains the mainstay of
most aggressive ICP management protocols. Mannitol causes the kidneys,
and hence the body, to lose water since mannitol is a large molecule
that is not absorbed in the distal renal tubule; the net effect is to
rapidly reduce swelling from edema. Each dose of mannitol is only
effective for a few hours, so dosing needs to be repeated frequently.
Mannitol cannot be used in patients with kidney failure, and hypertonic
saline solutions are a more appropriate choice in these patients.
In
patients whose ICP still remains refractory to treatment despite the
measures described above, a variety of third-line approaches are used,
including neuromuscular blockade, barbiturate coma, and induced
hypothermia. Rigorous evidence-based comparisons of these third-line
techniques do not exist and therefore institutional and individual
physician preference guides choice of strategy.
Intravenous
corticosteroids were previously used in an attempt to reduce cerebral
edema in patients with ICH. Although corticosteroids are likely
effective in reducing edema in some settings, such as with brain tumors,
randomized trials have established no role for these agents in ICH, and
their use may even be associated with worse outcome9.
In
some patients with ICH and high ICP refractory to medical therapy,
surgical hemicraniectomy may be indicated. This approach removes a large
portion of the skull so that the brain can swell outward, thereby
relieving pressure. While hemicraniectomy has been proven to be
effective in young patients with large ischemic strokes, its role in ICH
remains unclear and further studies are needed.
Prevention of Secondary ICH
Preventing
recurrent ICH is an important element of ICH management following the
acute period. Since hypertension is the single biggest risk factor for
ICH, controlling blood pressure to normal levels on discharge from the
hospital is imperative. Reduction of ICH risk with appropriate blood
pressure control has been demonstrated in multiple, large hypertension
trials10. Smoking, heavy alcohol use, and abuse of illicit
drugs such as cocaine and methamphetamines should be avoided as they
each contribute to an increased risk of ICH. Underlying lesions such as
vascular malformations and brain tumors that lead to ICH are usually
treated with surgery, radiation, or endovascular techniques after
hemorrhage; removal of these lesions, when possible, prevents recurrent
ICH.
1. Morgenstern LB,
Hemphill JC 3rd, Anderson C, et al. Guidelines for the management of
spontaneous intracerebral hemorrhage: a guideline for heath care
professionals from the American Heart Association/American Stroke
Association. Stroke. 2010;41:2108-2129.
2. Hemphill
JC, 3rd, Bonovich DC, Besmertis L, Manley GT, Johnston SC. The ICH
score: a simple, reliable grading scale for intracerebral hemorrhage. Stroke. 2001;32(4):891-897.
3. Delcourt
C, Huang Y, Wang J, et al. The second (main) phase of an open,
randomised, multicentre study to investigate the effectiveness of an
intensive blood pressure reduction in acute cerebral haemorrhage trial (INTERACT2). Int J Stroke. 2010;5(2): 110-6.
4. Baker
RI, Coughlin PB, Gallus AS, Harper PL, Salem HH, Wood EM. Warfarin
reversal: consensus guidelines, on behalf of the Australasian Society of
Thrombosis and Haemostasis. Med J Aust. 2004;181(9):492-497.
5. Davis SM, Broderick J, Hennerici M, et al. Hematoma growth is a determinant of mortality and poor outcome after intracerebral hemorrhage. Neurology. 2006;66(8):1175-1181.
6. Mayer SA, Brun NC, Begtrup K, et al. Recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med. 2005;352(8):777-785.
7. Mendelow
AD, Gregson BA, Fernandes HM, et al. Early surgery versus initial
conservative treatment in patients with spontaneous supratentorial
intracerebral haematomas in the International Surgical Trial in
Intracerebral Haemorrhage (STICH): a randomised trial. Lancet. 2005;365(9457):387-397.
8. Stocchetti N, Maas AI, Chieregato A, van der Plas AA. Hyperventilation in head injury: a review. Chest. 2005;127(5):1812-1827.
9. Poungvarin N, Bhoopat W, Viriyavejakul A, et al. Effects of dexamethasone in primary supratentorial intracerebral hemorrhage. N Engl J Med. 1987;316(20):1229-1233.
10. Perry
HM, Jr., Davis BR, Price TR, et al. Effect of treating isolated
systolic hypertension on the risk of developing various types and
subtypes of stroke: the Systolic Hypertension in the Elderly Program
(SHEP). Jama. 2000;284(4):465-471.
11. Mayer
SA, Brun NC, Begtrup K, et al. Efficacy and safety of recombinant
activated factor VII for acute intracerebral hemorrhage. N Engl J Med. 2008;358(20):2127-37.